Wildlife research – Are animal experiments necessary for species preservation?
They represent only a very small percentage of all animal experiments, and yet they are sometimes used to defend animal experiments in general: experiments on animals for the purpose of species preservation (1, 2). While the vast majority of animal experiments are kept strictly in secret and genuine images and footages can be accessed only through undercover investigations, animal experiments in species-preservation are handled almost openly.
Hardly any wildlife documentary is without scenes showing wild animals being captured to be measured, tagged or fitted with transmitters. Blood or tissue samples are taken, rings placed on legs, clips on wings, collars hung around necks or backpacks strapped on. These manipulations appear harmless to viewers, and after all the research is said to benefit species preservation — thus the animals.
This article aims to show that such animal experiments — and by definition these interventions are experiments — are by no means indispensable for species preservation, and that they often critically impair the individual animals involved. Furthermore, there are numerous innovative methods available that cause no harm to animals and can yield meaningful results.

Publicly visible animal experiment: A grey goose with a huge neck collar in a park in Braunschweig, Germany. ©DAAE
The importance of species preservation
The loss of species, also known as the biodiversity crisis, describes the rapid disappearance of animal and plant species on Earth. In recent decades, species loss has accelerated dramatically. It is estimated that extinction today occurs up to 1,000 times faster than the natural average (3). The main causes are human activities such as habitat destruction, environmental pollution, climate change and the excessive use of natural resources.
Studying the way of living and behaviour of wild animals is essential in order to understand the ecosystems in which they live and to implement protective measures. Equally important is studying animals’ adaptability to new or altered habitats, whether due to climate change or human interventions, so that appropriate measures for their protection can be taken.
Species preservation aims to preserve the diversity of animal and plant species on our planet. This biodiversity is crucial for the balance and stability of our ecosystems. Thus, species preservation is not only significant for nature but also for us humans.
Number of animal experiments in the area of wildlife research / species preservation
In Germany the number of animals recorded for this area varies significantly from year to year. Regarding the percentage, it is only a small part of all animal experiments. However, the statistics only cover vertebrates and cephalopods. Insects, crustaceans, worms and other invertebrates are not recorded.
|
2018 |
2019 |
2020 |
2021 |
2022 |
2023 |
|
|
Mice |
6,351 |
2,444 |
1,744 |
9 |
8 |
6 |
|
Chicken |
0 |
0 |
150 |
629 |
1,878 |
0 |
|
Other birds |
108 |
232 |
495 |
345 |
645 |
769 |
|
Reptiles |
0 |
0 |
66 |
44 |
0 |
0 |
|
Frogs |
0 |
0 |
0 |
990 |
0 |
0 |
|
Other Amphibia |
651 |
0 |
0 |
0 |
0 |
0 |
|
Salmon, trout an others |
6,881 |
0 |
0 |
14,320 |
1,552 |
5,398 |
|
Other fish |
152,212 |
20,865 |
10,109 |
5,646 |
9,656 |
|
|
Other animals |
97 |
46 |
260 |
131 |
255 |
507 |
|
Total |
14,088 |
154,934 |
23,580 |
26,577 |
9,984 |
16,336 |
|
Percentage |
0.66% |
7.03% |
1.2% |
1.4% |
0.58% |
1.1% |
Table 1: Number of animals used in animal experiments in Germany for the purpose of ‘species preservation’ and percentage of the total number (4).
Apart from a particularly high use of fish in 2019, between 10,000 and 26,000 animals were used for species-preservation in the past five years. In the EU and Norway in 2022 it was nearly half a million animals, which corresponds to 5.85% of the total number (5).
Capture of wild animals
Typically, capturing is the prerequisite for subsequent data collection. Measuring size and weight, taking blood and tissue samples, and attaching tags or transmitters are only possible if the animal is first caught. This happens in birds, bats and fish with various nets and traps. Other animals are caught in live traps or with snares. Fish are sometimes also caught by angling. For larger animals, anaesthetising darting fired from a distance is often used — partly for safety reasons for the humans involved.

Birds suffer from fear and panic when being captured. ©sevaljevic AdobeStock_233278426
In contrast to domestic animals, wild animals are not accustomed to human proximity or movement restriction. Numerous studies show that capture of wild animals can lead to substantial physical and psychological stress — regardless of whether anaesthesia is used. These damages range from injuries and long-term health issues and behavioural changes to death (6-11).
For example, grizzly bears (Ursus arctos) and American black bears (Ursus americanus) which were captured using helicopter-darting, barrel traps or foot snares showed reduced movement behaviour after capture, which only normalised after 3–6 weeks. Bears caught twice fared worse physically than bears caught only once (6).
The frequency of capture ranges from one-time for studies on species distribution to repeated captures over days, weeks, months or years — for measuring energy use or later retrieval of transmitters. That capture frequency influences animals is shown also in another example: wild reindeer (Rangifer tarandus platyrhynchus) when captured once, showed a short-term increase in the stress hormone cortisol; but multiple captures (4 times in 6 weeks) led female animals to have fewer offspring in the following summer (7).
Among some of the bears caught via foot snare (see above), severe physical damage occurred — they developed so-called “capture myopathy.” This muscle disease caused by capture stress was first described in 1964 in Hunter antelopes (Beatragus hunteri) and has since become a worldwide problem in all capture and relocation operations of wild animals (8).
Within hours or a few days a shock syndrome can appear. The animals show unstable gait, apathy, trembling, lameness up to paralysis; death often occurs due to kidney failure. Primarily affected are various mammals including marine mammals such as whales and dolphins, as well as birds. Fish, amphibians and reptiles are affected in individual cases. It has also been observed that animals survive the first capture well, but on the second they develop capture myopathy and die (8).
Triggers appear not only to be the stress of immobilization, but also noises of cars or helicopters, human proximity and manipulations such as measurements and attaching transmitters. With measures like sedatives, rest and cooling attempts are made to minimize risk of capture myopathy, but it still occurs repeatedly. Many examples exist in the literature. For instance, in Spain four little bustards (Tetrax tetrax) were caught with foot snares, to strap backpacks with transmitters onto them. One bird died after a day because it entangled a leg in the straps, the other three had difficulties walking and flying and died within 5-8 days of capture myopathy (9).
Proximity to humans and being handled even affect butterflies. Thus larvae, pupae and adult stages of the monarch butterfly showed elevated heart rates after 3-minute gentle handling (10).
The stress experience due to capture and release in wild animals can also lead to dramatic late consequences. There are numerous reports according to which animals could less effectively defend against predators, starved because they ate less, reproduced less or died from infections due to weakened immunity (11).
Frequent invasive research methods in wild animals
1. Samples of blood, tissue and stomach content
Blood and tissue samples are taken from wild animals to determine their genetic status and various metrics such as blood sugar, hormone levels or content of toxic substances like pesticides or heavy metals. In larger animals a blood sample can be taken via puncture of a vein in the legs, wings, neck or tail. But in small animals blood sampling is difficult and mutilations are accepted. In turtles and small birds such as hummingbirds toe-nails may be cut off to obtain a drop of blood (12). If at the same time a marking is to be done, e.g., in frogs and lizards whole toes (13) may be clipped or in fish one or more fins cut off (14).
To study the diet composition of animals, they are either killed or caught and their stomach flushed. For example, in unaesthetised frogs a tube is inserted through the mouth and oesophagus into the stomach and water is pumped in with a syringe. The water with stomach contents emerging from the mouth is collected. This is repeated until no stomach content is flushed out (15).
2. Marking
Animals are marked in various ways so that individual animals can be distinguished during observation and repeated capture. There are less traumatic methods like fur‐cuts (individual patterns cut in the fur) or colouring of fur or body parts (16). But many invasive marking methods are common that cause injuries and mutilations of animals (16,17). In all cases the traumatic experience of capture is the prerequisite for the subsequent marking.
2.1. Mutilations
Toe-clipping (i.e., cutting off toes) in differing positions and numbers is standard in “laboratory” mice under 7 days. Among wild living mice it is rather the exception, but still happens (17). In some mouse species this leads to a low recapture rate (17), suggesting that the animals associate capture with the pain of mutilation. By contrast, toe-clipping in amphibians and lizards as well as fin-clipping (cutting off fins) in fish is a usual marking method (14).
2.2. Branding
Branding of cattle and horses with a hot iron has a centuries-old tradition. Burns of third degree are inflicted to ensure permanent marking through scar formation. That this procedure is connected with enormous pain — even over long time periods — is well documented. For example, foals often showed pain symptoms lasting weeks and were behaviourally impaired (18). In wildlife research hot-branding is used with different seal species. Among Steller sea lions (Eumetopias jubatus) behavioural changes were observed in the first three days after branding. In harbour seal pups (Phoca vitulina) the wound did not heal after 9-10 weeks, and in southern elephant seals (Mirounga leonina) healing took up to a year (19).
2.3. Tags
A common method of identification is attaching plastic or metal tags with an individual number. Typical body parts where tags are placed are ears, wings and fins. Capturing and immobilization of the animals is a prerequisite. When attaching the tag a piece of tissue is often punched out, which is associated with corresponding pain. The subsequent impairments range from injuries of the marked body parts, altered behaviour, reduced food intake up to increased mortality (20-25).
For example, vultures (Gyps coprotheres) fitted with wing tags flew slower, less often and covered shorter distances than leg-banded birds (20). Magnificent frigatebirds (Fregata magnificens) marked on the wings showed poorer breeding success (21). An analysis of prey remains of peregrine falcons (Falco peregrinus) found that unusually many Montagu’s harriers (Circus pygargus) and hen harriers (Circus cyaneus) fitted with wing tags were predated upon. The result suggests that tags may attract attention from predators, which leads to increased predation rate and thus increased mortality (22).
Especially for many aquatic living animals, a streamlined body shape is vital for survival. A tag attached by humans increases drag in the water and leads to impaired swimming. This in turn affects food intake and makes the animal easier prey for predators. For instance, tagged Magellanic penguins (Spheniscus magellanicus) had to swim longer distances for daily food intake than non-tagged animals during years with food scarcity and thus expended more energy (23). In tagged Adélie penguins (Pygoscelis adeliae) injuries of the wings and a reduced survival rate of 24% were observed (24). A computer simulation showed that a device mounted on the back of a grey seal (Halichoerus grypus) increased water drag by 12%, which suggests altered swimming behaviour (25).

Adélie penguins: wing marks increase drag and make swimming more difficult, thus hindering food intake. ©Katie Dugger, USGS
2.4. Transmitters
Animals have been transmitter-tracked for decades to follow their home range and migration movements.
There are three tracking techniques:
- Very-High-Frequency radio-tracking (VHF)
- Satellite tracking
- Global Positioning System (GPS).
VHF has been standard since the 1960s in wildlife research. A transmitter attached to the animal sends signals on a predefined radio frequency at regular intervals. With antenna and receiver, based on the signal frequency and the time between two signals the position of the animal can be determined.
Satellite tracking, used since the 1980s, sends signals from a transmitter on the animal to certain satellites, which in turn are received on earth.
GPS technology has been used in wildlife research since the early 1990s. Signals from at least three satellites are received, from which the position is calculated. Although GPS units are small and light, they present a disadvantage in that the data are stored in the GPS device. To read them out the animal must be captured a second time or even repeatedly. GPS and satellite technology may also be combined to continuously obtain data, but this increases the size and weight of the device (26).
Typically, batteries or small solar panels are used as power source. There is the possibility that the transmitter falls off the animal, e.g., after a predetermined time or when the battery is empty — thus avoiding another capture.

Eagle fitted with a transmitter-backpack. ©belizar AdobeStock_79454631
The transmitters are attached externally (via a collar or backpack) or glued on. Or they are implanted under the skin or into the abdominal cavity. For such an operation the animals must be anaesthetised. The duration of transmitter use may vary greatly depending on the research question – from a few days to many years.
The problems arising from transmitter use are manifold. One study from the USA reports that sparrows tangled body parts in the straps of the transmitter or became stuck in vegetation (27). Eastern phoebes (Sayornis phoebe) threw their chicks from the nest in an attempt to remove the transmitter from their young. In other sparrows (Ammospiza caudacuta) the parents abandoned their chicks or fed only the non-transmitter-fitted ones (27). In mantled howler monkeys (Alouatta palliata) serious skin and muscle injuries were found around the collar area (28).

The weight of a transmitter collar affects zebra behaviour while grazing. ©PACO COMO AdobeStock_134126186
The size and weight of the transmitter and the method of attachment play a role. Recommended weight ranges from 0.7% up to 9% of body weight, with an average under 5% (29). In zebras (Equus burchelli antiquorum) in Botswana it was observed that even a small weight difference had major behavioural impact: animals with a heavier GPS collar (1.8 kg) covered only half the distance when grazing compared to zebras with a lighter collar (1.2 kg), despite both collars being well below the lowest guideline threshold (0.4% vs 0.6% of body weight respectively) (29).
Especially dramatic can be the effects for animals into whose abdominal cavity transmitters are implanted. Among 305 dead or living Scandinavian brown bears fitted with VHF transmitters up to 13 years post-implantation, severe pathological changes such as inflammation and adhesions in the abdominal cavity were found. The batteries were in some cases corroded or leaked. Two bears died from a short-circuit of the batteries (30).
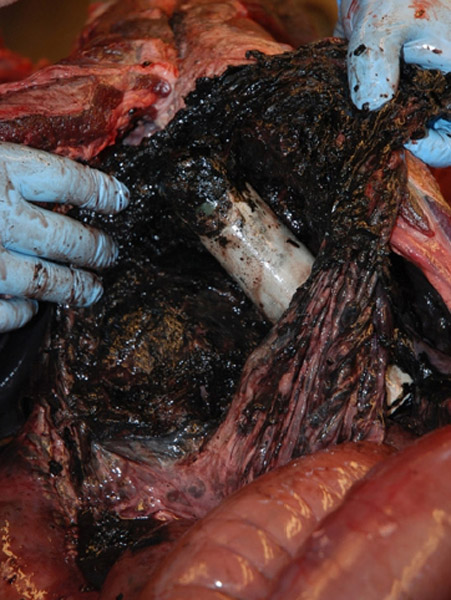
Serious pathological changes caused by a transmitter in a bear's abdominal cavity. Reference: Arnemo. Frontiers in Veterinary Science 2018; 5: 252
Severe immune reactions were also found in European beavers (Castor fiber) and channel catfish (Ictalurus punctatus) that had devices or temperature/heart monitors implanted into their abdominal cavity. The transmitters and monitoring devices were rejected by the body and expelled through the intestines or the operation wound (31).
Because of their body shape, for snakes surgical implantation of devices into the abdominal cavity is standard. However, a number of problems may occur, from anaesthesia risk and wound infections to negative effects on growth and reproduction (31). Increasingly external devices are used in snakes, e.g., glued with tape or sewn on. Negative effects included changed behaviour and movement patterns, skin and scale injuries, and even death (32).
3. Killing
For species identification and for museum collections animals were commonly killed in the past. In recent decades more non-lethal methods are used, yet there are still vehement defenders of killing animals for these purposes. Especially amphibians, birds and small rodents are commonly killed for DNA analysis (33). Also, insects, spiders, scorpions and other invertebrates fall victim to species identification. To identify species diversity on a meadow, for instance, all organisms in a certain area may be collected with a net and preserved in alcohol.

Collections containing countless animals preserved in alcohol are standard at many universities. © DAAE
Legal regulations
“Research for the purpose of species preservation” is listed in the German Animal Welfare Act as one of the permissible purposes for animal experiments. Animal experiments on vertebrates and cephalopods require approval by the competent authority, usually the regional government (34). Animal experiments in the field of wildlife research are therefore regulated in the same way as other animal experiments. However, an additional special permit is required by the competent authority when animals are “taken from nature” (35). Invertebrate animals (other than cephalopods) are not covered by these legal requirements — meaning they can be used, tortured and killed without legal consequences.
Animal-experiment-free wildlife research
On the website “3R’s Principles in Wildlife Research” by Marina A. Zemanova a wealth of animal-experiment-free research methods sorted by research objective and animal class is presented (36). Researchers can inform themselves via numerous studies and instructional videos.
Unfortunately, the author limits herself to the 3R principle, which besides replacement also includes reduction and refinement of animal experiments. The concept developed in the 1950s positions the animal experiment as a method that is not questioned and wants only a modification of the existing system. Our association DAAE demands, however, the abolition of all animal experiments for ethical and scientific reasons (37). So, unfortunately, some studies on Zemanova’s website merely represent a refinement — for instance a swab from the mouth of caught fish (38). Also stomach-flushing of frogs and experimentally inducing stress in rabbits via fox scent to measure cortisol in faeces are listed as non-invasive methods here (39). Nevertheless, the website can serve as an extremely important resource for animal-experiment-free wildlife research.
1. Faecal samples
Faecal samples carry a lot of information. For example, species can be determined via DNA (40), physiological parameters such as sex and stress hormones (41), diet components (42), absorbed pollutants like heavy metals, toxins (43) or microplastics (44), as well as presence of parasites (45) and viruses (46) can be investigated.
2. Hair and other samples
Hair can be used for species identification via DNA analysis (47) or examined for heavy metals such as lead and mercury (48). To obtain hair samples hair-traps are used: sticky tapes near hamster burrows (49) or otter couches (50), power poles fitted with barbed wire where bears rub (51), or for territory marking by lynx favourite trees (47). Also moulted hairs of sea lions (48), hairs found in wildlife underpasses (52) or hairs of road-kill animals (53) can be analysed.
Likewise moulted feathers from birds can detect DNA (54) and heavy metals (55).
DNA analysis is now so advanced that a genetic profile can be determined not only from faeces, hairs and feathers but also from urine (56), eggshells (57), shed antlers (58), shed skin from lizards (59), collected skin pieces from whales (60), and placentas of seals (61). Even spider webs (62) and snail slime (63) contain sufficient DNA trace for species identification. For animals that move very slowly or not at all (e.g., mussels) swab samples may be used (64). Skin particles of manta rays can be sampled underwater with a toothbrush (65), and with a lice-comb on a telescopic pole skin parasites of sea lions can be collected (66).
3. Environmental examination
Animals leave traces in their environment. For example, the genetic code can be traced in water not only from aquatic animals such as fish (67) and whales (68), but also from land animals drinking water (69) or temporarily swimming in it (70). Earthworms leave DNA traces in soil (71), insects (72), nectar-drinking bats and hummingbirds (73) on the flowers they visit. Via a telescopic pole or drone the blow of whales and dolphins can be captured – this not only determines the genetic code (74) but also provides information about health by examining bacteria (75), pollutants such as chemicals (76), hormones (77) and the microbiome (78).

Bats leave DNA traces on the flowers they visit. ©FotoRequest_AdobeStock_598449622
Saliva on leftover food is also very useful. This can include fruit pecked by birds (79), leaves chewed by bonobos (80), salmon remains left behind by bears (81), or even tools made by chimpanzees for termite fishing (82).
Traces of the genetic profile can even be found in the air, for example at bat roosts (83), in footprints in the snow (84), and in scent markings used to define territories (85).
4. Photo-ID / Face recognition / AI
Instead of tagging or transmitter-fitting animals, modern computer-based recognition methods can be used – so-called Photo-ID – to identify individual animals. Even with animals that look very alike or whose way of life leaves little visible, individual assignment is possible. For example: whales can be recognised by their fluke or dorsal fin (86); crocodiles by the pattern of their tail-scales (87); manatees by their individual scar pattern (88); polar bears via their whisker pattern (89). For many animals fur colour and pattern is also individual. The photos required can be obtained via camera-traps, drones or unmanned aerial vehicles without disturbing the animals.

Whales can be identified by the individual shape of their fluke. © ÄgT
Photos can also provide information about diet composition (90), parasite load (91) and biometric body measurements (92).
The Photo-ID technique can also be used for foot, hoof or claw impressions (93) to track movement patterns of individual animals. Bears (94) and primates (95) can be identified via face-recognition software, using deep learning (a machine-learning method). Using deep learning even flying insects can be identified via their flight patterns (96).
In reef fish, AI-assisted high-resolution 3D-tracking was used to gain detailed insight into movement patterns and energy expenditure of the animals (97).
Modern photogrammetry and 3D-scanning with high-resolution cameras can help investigate the anatomy of animals (98). For species identification, the calls, sounds and noises animals make can also serve — e.g., cricket chirps (99), bees/hornets flight-sounds (100) or ultrasound from bats (101).
„It was a mistake to equip them with instruments“
The documentary “Patrick and the Whale” shows how knowledge about animals’ behaviour can be gained with patience and respect without harming the animals — and also makes clear how not to do it. The film tells the story of an extraordinary relationship between sperm whales and the American underwater filmmaker Patrick Dykstra. Over more than a decade Dykstra swam and dived with sperm whales and documented their fascinating behaviour in breathtaking footage. Over long periods two female sperm whales built up a trust relationship with him and let him share their hidden world. Dykstra wanted to exploit this and fit one of the whales with a camera. With whale lady “Dolores” things went fine — she tolerated the camera, attached with suction cups, which was planned to fall off after eight hours. But “Can Opener” showed clear defensive reactions when he attempted to attach the camera, and later she kept her distance of only a few metres from him. Dykstra sums up: “It was a mistake to equip them with instruments and treat them like a scientific experiment.” (102)
Does the end justify the means?
In wildlife research the protection of species and biodiversity is in the foreground. The welfare of the individual animal regularly recedes into the background. Only in this way is it possible to explain that for decades animals have been intentionally exposed to capture and various manipulations — often into the greatest suffering. Death of the animals is either planned or at least accepted. A rethink seems hardly to be making progress.
A survey among ecology researchers found that more than a quarter of them kill animals for research purposes; a further 18 % use exclusively invasive methods that can lead to damage to the animals; roughly a third use both invasive and non-invasive techniques; and only 22 % reported using only non-invasive methods. As hurdles for the use of non-invasive investigations, the most commonly cited were financial restrictions and lack of awareness (103).
Besides this alarming obvious indifference toward the suffering of the individual animal, the scientific relevance of the data obtained must also be questioned. If a difference of 600 g in the weight of a collar on a 320 kg zebra has massive behavioural impact (29) then one must ask whether even the lightest collar and the “gentlest treatment” do not cause behavioural change and thus miss the actual objective of the experiment.
The call for the abolition of all animal experiments rightly includes wildlife research. It exposes animals to suffering and death, although even in this field modern non-harmful research methods are available in large number. Animal experiments in wildlife research are unjustified and must — just like all other animal experiments — be abolished.
02/06/2025
Dr Corina Gericke DVM
Further information
The 3Rs principles in wildlife research https://3RsWildlife.info
References
- Tierversuche für den Artenschutz, Tierversuche verstehen. 15.01.2024 [retrieved 31.05.2025]
- Richter S.H. et al. Animal research revised – the case of behavioural studies. Trends in Ecology & Evolution 2024; org/10.1016/j.tree.2024.11.014
- Pimm S.L. et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014; 344(6187): 1246752. DOI: 10.1126/science.1246752
- Bundesinstitut für Risikoforschung (BfR): Verwendung von Versuchstieren im Jahr 2022
- ALURES – EU statistics database [retrieved 31.05.2025]
- Cattet M. et al. An evaluation of long-term capture effects in ursids: implications for wildlife welfare and research. Journal of Mammalogy 2008; 89(4):973-990
- Trondrud L.M. et al. Stress responses to repeated captures in a wild ungulate. Nature.com. Scientific reports 2022; 12:16289
- Breed D. et al. Conserving wildlife in a changing world: Understanding capture myopathy—a malignant outcome of stress during capture and translocation. Conservation Physiology 2019; 7: 10.1093/conphys/coz027
- Marco I. et al. Capture myopathy in little bustards after trapping and marking. J Wildl Dis 2006; 42(4):889-9
- Davis A.K. et al. Evaluating cardiac reactions of monarch butterflies to human handling across three life stages. The Journal of Lepidopterists’ Society 2020; 74: 43-50
- Dickens M.J. et al Stress: An inevitable component of animal translocation. Biological Conservation 143 (2010) 1329–1341
- Johnson J.D. Nail trimming for blood collection from desert tortoises, Gopherus agazzizii: Panel Summary. Journal of Herpetological Medicine and Surgery 2006; 6(2):61-62
- Grafe T.U. et al. Putting toe clipping into perspective: a viable method for marking anurans. Journal of Herpetology 2011; 45(1):28-35
- Eriksen T.B. Should fin clipping be used as a method for identification of fish? Norwegian School of Veterinary Science 2011
- Solé M. et al. Stomach-flushing for diet analysis in anurans: An improved protocol evaluated in a case study in Araucaria forests, southern Brazil. Studies on Neotropical Fauna and Environment 2005; 40(1): 23-28
- Bundesamt für Umwelt, Schweizerische Eidgenossenschaft: Fang, Markierung und Beprobung von freilebenden Wildtieren. 2018
- Deutsch M. Methoden zur Markierung von Kleinsäugern im Freiland – eine Übersicht. Beiträge zur Jagd- und Wildforschung 2015; 40: 275-287
- Bohnet W. Stellungnahme der Tierärztlichen Vereinigung für Tierschutz (TVT) zur Kennzeichnung von Pferden (Equiden) mittels Heißbrand und/oder Transponder. TVT, 20.08.2010
- Walker K. et al. Behavioural responses of juvenile Steller sea lions to hot-iron branding. Applied Animal Behaviour Science 2010; 122(1):58-62
- Curk T. et al. Wing tags severely impair movement in African Cape Vultures. Curk et al. Anim Biotelemetry 2021; 9:11
- Trefry S.A. Wing marker woes: a case study and meta-analysis of the impact of wing and patagial tags. Journal of Ornithology 2013; 154: 1-11
- Zuberogoitia I. et al. Standing out from the crowd: are patagial wing tags a potential predator attraction for harriers (Circus spp.)? Journal of Ornithology 2012; 153: 985–989
- Wilson R.P. Pushed to the limit: food abundance determines tag-induced harm in penguins. Animal Welfare 2015; 24(1): 37-44
- Jackson S. et al. The potential costs of flipper-bands to penguins. Functional Ecology 2002; 16: 141–148
- Hazekamp A.A.H. et al. Flow simulation along a seal: the impact of an external device. Eur J Wildl Res (2010) 56:131–140
- Mech L.D. and Barber S.M. A Critique of wildlife radio-tracking and its use in the U.S. national Parks. 6.2.2002
- Hill J.M. and Elphick C.S. Are grassland passerines especially susceptible to negative transmitter impacts? Wildlife Society Bulletin 2011; 35(4): 362-367
- Hopkins M.E. et al. Adverse Effects of Ball-Chain Radio-Collars on Female Mantled Howlers (Alouatta palliata) in Panama. Int J Primatol 2016; 37: 213–224
- Brooks C. et al. Effects of global positioning system collar weight on zebra behavior and location error. Wildlife Management 2010: doi.org/10.2193/2007-061
- Arnemo J.M. et al. Long-term safety of intraperitoneal radio transmitter implants in brown bears (Ursus arctos). Frontiers in Veterinary Science 2018; 5: 252
- Mayer M. et al. Retention and loss of PIT tags and surgically implanted devices in the European beaver. BMC Veterinary research 2022; 18:219
- Christensen T. et al. External transmitter attachment in snakes: a systematic review of methods, efficacy, and impacts. Animal Biotelemetry 2024; 12:14
- Waeber P.O. et al. On specimen killing in the era of conservation crisis - A quantitative case for modernizing taxonomy and biodiversity inventories. PLOS One 2017; 12(9): e0183903
- Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206, 1313), das zuletzt durch Artikel 2 Absatz 20 des Gesetzes vom 20. Dezember 2022 (BGBl. I S. 2752) geändert worden ist
- Tierschutz-Versuchstierverordnung vom 1. August 2013 (BGBl. I S. 3125, 3126), die zuletzt durch Artikel 1 der Verordnung vom 11. August 2021 (BGBl. I S. 3570) geändert worden ist
- Zemanova M.A. The 3Rs principles in wildlife research [retrieved 31.05.2025]
- Ärzte gegen Tierversuche. Positionspapier zum 3R-Konzept – Tierversuche reduzieren, ersetzen oder abschaffen. 10.12.2024 [retrieved 31.05.2025]
- Colussi S. et al. Buccal swab: A tissue sampling method for refinement of experimental procedures involving rainbow trout. Journal of Applied Ichthyology 2017; 33: 515-519
- Monclús R. et al. Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 2006; 16: 25-29
- Manning J.A. et al. Scat as a source of DNA for population monitoring. Ecology and Evolution 2022; 12: e9415
- Yang L.L. et al. Non-invasive determination of fecal steroid hormones relating to conservation practice in giant panda (Ailuropoda melanoleuca). Animal Biology 2011; 61: 335-347
- McInnes J.C. et al. High occurrence of jellyfish predation by black‐browed and Campbell albatross identified by DNA metabarcoding. Molecular Ecology 2017; 26: 4831-4845
- Afonso E. et al. Is the lesser horseshoe bat (Rhinolophus hipposideros) exposed to causes that may have contributed to its decline? A non-invasive approach. Global Ecology and Conservation 2016; 8: 123-137
- Desclos‐Dukes L. et al. Using a non‐invasive technique to identify suspected microplastics in grey seals (Halichoerus grypus) living in the western North Sea. Veterinary Record 2022: e1484
- Napoli E. et al. Survey on parasitic infections in wildcat (Felis silvestris silvestris Schreber, 1777) by scat collection. Parasitology Research 2016; 115: 255-261
- Briscoe A.G. et al. High-throughput sequencing of faeces provides evidence for dispersal of parasites and pathogens by migratory waterbirds. Molecular Ecology Resources 2021; 22: 1303-1318
- Schmidt K. et al. Using scent‐marking stations to collect hair samples to monitor Eurasian lynx populations. Wildlife Society Bulletin 2006; 34: 462-466
- Andrade S. et al. Heavy metals in molted fur of the southern elephant seal Mirounga leonina. Marine Pollution Bulletin 2077; 54: 602-605
- Reiners T.E. et al. An optimized hair trap for non-invasive genetic studies of small cryptic mammals. European Journal of Wildlife Research 2011; 57: 991-995
- Pedroso N.M. et al. Non-invasive hair sampling of neotropical otters. Biota Neotropica 2018; 18: e20180579
- Karamanlidis A.A. et al. Noninvasive genetic studies of brown bears using power poles. European Journal of Wildlife Research 2010; 56: 693-702
- Clevenger A.P. et al. Piloting a non-invasive genetic sampling method for evaluating population-level benefits of wildlife crossing structures. Ecology and Society 2010; 15: 7
- Picone M. et al. Neonicotinoids and pharmaceuticals in hair of the red fox (Vulpes vulpes) from the Cavallino-Treporti peninsula, Italy. Environmental Research 2023; 228:115837
- Segelbacher G. Noninvasive genetic analysis in birds: testing reliability of feather samples. Molecular Ecology Notes 2022; 2: 367-369
- Dauwe T. et al. Variation of heavy metals within and among feathers of birds of prey: effects of molt and external contamination. Environmental Pollution 2003; 124: 429-436
- Hausknecht R. et al. Urine – a source for noninvasive genetic monitoring in wildlife. Molecular Ecology Notes 2007; 7: 208-212
- Shamblin B.M. et al. Loggerhead turtle eggshells as a source of maternal nuclear genomic DNA for population genetic studies. Molecular Ecology Resources 2011; 11: 110-115
- Venegas C. et al. Non-invasive genetic sampling of deer: a method for DNA extraction and genetic analysis from antlers. Gayana 2020; 84: 67-74
- Horreo J.L. et al. Skin sheds as a useful DNA source for lizard conservation. Phyllomedusa: Journal of Herpetology 2015; 14: 73-77
- Neveceralova P. et al. Population changes in a whale breeding ground revealed by citizen science noninvasive genetics. Global Ecology and Conservation 2022; 37: e02141
- Valtonen M. et al. Genetic monitoring of a critically-endangered seal population based on field-collected placentas. Annales Zoologici Fennici 2015; 52: 51-65
- Xu C.C. et al. Spider web DNA: a new spin on noninvasive genetics of predator and prey. Plos One 2015; 10: e0142503
- Kawai K. et al. A non-invasive technique for obtaining DNA from marine intertidal snails. Journal of the Marine Biological Association of the United Kingdom 2004; 84: 773-774
- Henley W.F. et al. Non-invasive method to obtain DNA from freshwater mussels (Bivalvia: Unionidae). Journal of Shellfish Research 2006; 25: 975-977
- Kashiwagi T. et al. Evaluating manta ray mucus as an alternative DNA source for population genetics study: underwater-sampling, dry-storage and PCR success. PeerJ 2015; 3: e1188
- Ebmer D. et al. Antarctophthirus microchir infestation in synanthropic South American sea lion (Otaria flavescens) males diagnosed by a novel non-invasive method. Parasitology Research 2019; 118: 1353-1361
- Yang J. et al. Small changes make big progress: A more efficient eDNA monitoring method for freshwater fish. Environmental DNA 2023; 5: 363-374
- Székely D. et al. Environmental DNA captures the genetic diversity of bowhead whales (Balaena mysticetus) in West Greenland. Environmental DNA 2021; 3: 248-260
- Ushio M. et al. Environmental DNA enables detection of terrestrial mammals from forest pond water. Molecular Ecology Resources 2017; 17: e63-e75
- Reinhardt T. et al. Monitoring a loss: Detection of the semi‐aquatic crocodile lizard (Shinisaurus crocodilurus) in inaccessible habitats via environmental DNA. Aquatic Conservation: Marine and Freshwater Ecosystems 2019; 29: 353-360
- Bienert F. et al. Tracking earthworm communities from soil DNA. Molecular Ecology 2012; 21: 2017-2030
- Thomsen P.F. et al. Environmental DNA metabarcoding of wild flowers reveals diverse communities of terrestrial arthropods. Ecology and Evolution 2019; 9: 1665-1679
- Walker F.M. et al. Endangered nectar-feeding bat detected by environmental DNA on flowers. Animals 2022; 12: 3075
- Raudino H.C. et al. Challenges of collecting blow from small cetaceans. Ecosphere 2019; 10: e02901
- Acevedo‐Whitehouse K. et al. A novel non‐invasive tool for disease surveillance of free‐ranging whales and its relevance to conservation programs. Animal Conservation 2010; 13: 217-225
- Cumeras R. et al. Chemical analysis of whale breath volatiles: a case study for non-invasive field health diagnostics of marine mammals. Metabolites 2014; 4: 790-806
- Hogg C.J. et al. Determination of steroid hormones in whale blow: it is possible. Marine Mammal Science 2009; 25: 605-618
- Apprill A. et al. Extensive core microbiome in drone-captured whale blow supports a framework for health monitoring. mSystems 2017; 2: e00119-17
- Monge O. et al. Environmental DNA from avian residual saliva in fruits and its potential uses in population genetics. Conservation Genetics Resources 2020; 12: 131–139
- Ishizuka S. et al. Bonobos’ saliva remaining on the pith of terrestrial herbaceous vegetation can serve as non-invasive wild genetic resources. Primates 2019; 60: 7-13
- Wheat R.E. et al. Environmental DNA from residual saliva for efficient noninvasive genetic monitoring of brown bears (Ursus arctos). Plos One 2016; 11: e0165259
- Stewart F.A. et al. DNA recovery from wild chimpanzee tools. Plos One 2018; 13: e0189657
- Garrett N.R. et al. Out of thin air: surveying tropical bat roosts through air sampling of eDNA. PeerJ 2023; 11: e14772
- Kinoshita G. et al. Environmental DNA collected from snow tracks is useful for identification of mammalian species. Zoological Science 2019; 36: 198-207
- Malherbe G.P. et al. Genetic clues from olfactory cues: brown hyaena scent marks provide a non-invasive source of DNA for genetic profiling. Conservation Genetics 2009; 10: 759-762
- Franklin T. et al. Photo-identification of individual Southern Hemisphere humpback whales (Megaptera novaeangliae) using all available natural marks: managing the potential for misidentification. Journal of Cetacean Research and Management 2020; 21: 71-83
- Boucher M. et al. A tail of two crocs: coding tail-spot patterns for individual identification of American (Crocodylus acutus) and Morelet’s (Crocodylus moreletii) crocodiles. Mesoamerican Herpetology 2017; 4: 760-772
- Landeo-Yauri S.S. et al. Using small drones to photo-identify Antillean manatees: a novel method for monitoring an endangered marine mammal in the Caribbean Sea. Endangered Species Research 2020; 41: 79-90
- Anderson C.J.R. et al. Can whisker spot patterns be used to identify individual polar bears? Journal of Zoology 2007; 273: 333-339
- Naude V.N. et al. Using web-sourced photography to explore the diet of a declining African raptor, the Martial Eagle (Polemaetus bellicosus). The Condor: Ornithological Applications 2019; 121: duy015
- Barroso P. et al. Camera traps reveal a high prevalence of sarcoptic mange in red foxes from northern Spain. Research in Veterinary Science 2023; 166: 105098
- Ulikowski D. et al. In vivo calibration of juvenile crayfish body length and weight with a photographic-computer method. Fisheries & Aquatic Life 2009; 17: 89-93
- Alibhai S. et al. The challenge of monitoring elusive large carnivores: an accurate and cost-effective tool to identify and sex pumas (Puma concolor) from footprints. Plos One 2017; 12: e0172065
- Clapham M. et al. Automated facial recognition for wildlife that lack unique markings: A deep learning approach for brown bears. Ecology and Evolution 2020; 10: 12883-12892
- Crouse D. et al. LemurFaceID: a face recognition system to facilitate individual identification of lemurs. BMC Zoology 2017; 2: 2
- Kirkeby C. et al. Advances in automatic identification of flying insects using optical sensors and machine learning. Scientific Reports 2021; 11: 1555
- Lilkendey J. et al. Herbivorous fish feeding dynamics and energy expenditure on a coral reef: Insights from stereo-video and AI-driven 3D tracking. Ecology & Evolution 2024; 14: e11070
- Deakos M.H. Paired-laser photogrammetry as a simple and accurate system for measuring the body size of free-ranging manta rays Manta alfredi. Aquatic Biology 2010; 10: 1-10
- Jeliazkov A. et al. Large-scale semi-automated acoustic monitoring allows to detect temporal decline of bush-crickets. Global Ecology and Conservation 2016; 6: 208-218
- Kawakita S. et al. Automated classification of bees and hornet using acoustic analysis of their flight sounds. Apidologie 2019; 50: 71-79
- Mac Aodha O. et al. Bat detective – Deep learning tools for bat acoustic signal detection. Plos Computational Biology 2018; 14: e1005995
- Patrick and the Whale – eine außergewöhnliche Freundschaft. Dokumentarfilm, Österreich 2023
- Zemanova M.A. Making room for the 3R’s principles of responsible animal use in ecology: Potential issues identified through a pilot survey. European Journal of Ecology 2021; 7(2): 18-39
