Bipolar disorder: animal experiments and animal-free research
Bipolar disorder is among the most severe mental illnesses, significantly impacting the lives of those affected and those close to them. It is characterized by extreme mood swings, ranging from manic highs to depressive lows, and affect approximately 1 to 3% of the global population (1). Despite intensive research, the exact causes of bipolar disorder are still not fully understood. This complicates the development of effective treatment methods. Moreover, research into new therapies continues to rely heavily on so-called animal models. This article explains why animal experimentation is not a promising approach and which modern, human-focused methods are better suited.
Bipolar disorder is a mental illness characterized by extreme mood swings. Those affected experience alternating phases of mania (euphoric, excessively active mood) and depression (deep sadness and lack of drive). Manic episodes are often marked by heightened self-confidence, increased risk-taking, and insomnia, while depressive phases are frequently accompanied by feelings of worthlessness, hopelessness, and a loss of interest and activity. Between these episodes, individuals may experience a stable or “normally” fluctuating mood.
Available treatment options
Treatment for bipolar disorder typically involves a combination of medication and psychotherapy. Commonly used medications include mood stabilizers such as lithium, antipsychotics, and antidepressants. Cognitive behavioral therapy is often used as a complementary approach, aiming to promote self-regulation and improve understanding of one’s condition.
However, many patients do not respond fully or satisfactorily to treatment, highlighting the need for new and more precisely tailored therapies. To develop these, many researchers still use animal experiments.
Animal experiments
Animal experimentation related to bipolar disorder is mainly carried out on mice and rats. Researchers attempt to artificially induce symptoms of bipolar disorder in these animals—such as hyperactivity or anhedonia, which is a reduced ability to feel pleasure. However, the complex nature of bipolar disorder, which involves alternating manic and depressive episodes, cannot be fully replicated in any animal model (2). Instead, many researchers focus mainly on so-called "animal models" of mania, even though that only represents one part of the illness (2). The supposedly “manic” animals primarily show signs of hyperactivity, while symptoms like anhedonia—associated with depressive episodes—are also artificially induced.
To trigger these behaviors, animals are subjected to chronic stress or treated with specific drugs. There are also genetically modified animal models that display behaviors resembling manic states. The following section presents some examples of these types of experiments.
Induction of symptoms through chronic stress
Animals are repeatedly exposed to stress in order to provoke behaviors such as apathy or social withdrawal—reactions that are interpreted as parallels to depressive states in humans. Manic symptoms like hyperactivity can be triggered through stress, too. Common methods include social isolation, which is particularly distressing for social animals like mice and rats. The creativity shown by researchers in designing these stress models is as impressive as it is troubling.
For example, sleep deprivation is used to induce mania-like symptoms. In one such experiment, animals are placed on a small platform above water. If they fall asleep, they risk falling into the water, so they are forced to stay awake. After 72 hours of sleep deprivation the animals display behaviors such as hyperactivity, aggression, insomnia, and increased sexual activity—symptoms that researchers interpret as signs of mania (3).
Mania in humans can include aggression and restlessness. To mimic this in animals, researchers use a method called the “Resident-Intruder Test.” In this setup, a strange animal (the intruder) is placed in the cage of a previously isolated animal (the resident), which often leads to aggressive behavior such as biting or threatening gestures.
Some particularly elaborate animal experiments combine multiple stress factors. One “mouse model” of mania is based on exposing the animals to various unpredictable stressors over a period of three weeks. Each day, the mice are subjected to two randomly selected stressors from a pool that includes disruption of their day-night rhythm, sleep deprivation, bright light, loud noise, and electric foot shocks (4). For example, the light-dark cycle may be altered, the animals may be kept awake, exposed to strobe lights or 100 dB of noise (roughly equivalent to a chainsaw), the temperature may be raised to 45°C for 30 minutes, and they may receive electric shocks to their feet (4). In response to this ordeal, the animals become hyperactive and restless, lose interest in sweets, and show a disrupted sleep pattern - all of which are interpreted by researchers as signs of mania.
In similar experiments using different stressors, researchers attempt to mimic depression-like states. Over a period of three weeks, mice are randomly exposed to situations such as: restricted movement for four hours, wet bedding for 12 hours, cage shaking, being hung by the tail, forced swimming (see below), or having their cage tilted at a 45° angle for 12 hours. Again, two of these stressors are applied daily in a random order (4).
Pharmacological models
People with bipolar disorder often show elevated dopamine levels during manic episodes. In animal experiments, this is simulated by injecting amphetamines, which increase dopamine release in the brain. Mice or rats are typically given a dose of amphetamine to trigger excessive hyperactivity and other behavioral changes. In some cases, amphetamines are administered chronically over a longer period. After the drug is given, researchers observe the animals' behavior - particularly motor activity such as running or jumping - as an indicator of hyperactivity. In some studies, cognitive functions like memory and learning are also tested to examine the types of cognitive deficits that can occur in bipolar disorder. However, this model has clear limitations: the symptoms seen in animals often differ significantly from those in humans, and mania involves much more than just hyperactivity. Additionally, bipolar disorder is a chronic condition, while amphetamine treatments in animal studies are usually short-term or acute (5).
To better replicate human mania, some experiments combine amphetamines with other substances to trigger a wider range of symptoms. One example is the combination of amphetamine with chlordiazepoxide (CDP), a drug that normally has calming and anti-anxiety effects. Together, these substances are intended to provoke behaviors that researchers interpret as manic.
Another experiment uses a substance called ouabain, which is injected directly into the animals’ brains. Ouabain induces hyperactivity and is therefore considered a possible model for mania. However, its effects can last for up to seven days and cause significant damage to nerve cells in the brain (5).
Genetic models
Genetic studies have shown that changes in certain genes are linked to mental health conditions such as depression, anxiety disorders, and bipolar disorder. To better understand how individual genes influence behavior and brain function, researchers create "animal models" with targeted genetic modifications. This includes mutating, disabling, or adding specific genes.
Some of these models—such as transgenic mice—have been specifically developed to study manic behavior and mood swings. However, most genetically modified animals do not naturally alternate between manic and depressive states. Some do show mood fluctuations when exposed to changes in their environment or disruptions to their day-night rhythm (5).
One gene, known as Clock, has been associated with symptoms like manic episodes, insomnia, and a reduced need for sleep. To explore this genetic connection in an animal model, researchers developed the so-called ClockΔ19 mutant mouse. These mice carry a genetic mutation that disrupts the function of the Clock protein - a protein essential for regulating the body’s internal clock. As a result, their sleep-wake cycle and behavior are significantly altered, with disturbances that are often more extreme than those seen in humans with bipolar disorder.
In addition to this, scientific literature describes other genetically modified animal models for studying bipolar disorder (5). Using modern gene-editing technologies like CRISPR-Cas, researchers can now introduce mutations that have been linked to bipolar disorder in humans into mice.
Behavioral tests in animal experiments
The procedures for creating so-called animal models described above aim to replicate individual aspects of bipolar disorder in animals - such as hyperactivity (as a symptom of mania) or anhedonia (as a symptom of depression). In animal experiments based on these models, researchers often use behavioral tests to make these symptoms measurable and quantifiable. Some of these tests are described in more detail below.

In the Tail Suspension Test, mice are suspended by their tails. If they remain motionless for an extended period, this is interpreted as a sign of depressive behavior.
- In the Forced Swim Test (FST), also known as Behavioral Despair Test, mice or rats are placed in a cylinder filled with water, where they cannot stand and are unable to escape. The test typically lasts for 6 minutes. During this time, the duration of swimming and escape attempts is measured, as well as how long the animals remain motionless, floating on the surface. Longer periods of immobility are interpreted as signs of depression (6).
- In the Tail Suspension Test (TST), mice are suspended by their tails, preventing them from touching the ground. Extended periods of immobility are interpreted as despair and considered depressive-like behavior (6).
- In the Foot Shock Escape Test, electrical foot shocks are administered to mice through a grid they are sitting on, from which they cannot escape. In later trials, researchers observe whether the animals try to escape the shocks when given the opportunity. This test is used to assess learned helplessness.
- In the Sucrose Consumption Test, animals are offered two bottles: one with regular water and one with a sucrose solution. Reduced consumption of the sucrose solution is considered an indicator of depression (6).
- In the Open-Field Test, animals are placed in a test arena, and their movements are recorded. Reduced activity and spending more time near the walls are interpreted as depressive symptoms, while increased activity and exploration of the brightly lit center are interpreted as signs of a manic state.
In these tests, complex emotional states of chronically ill humans are reduced to simple behaviors of animals in acute stress situations. Additionally, interpretation is challenging, and it is difficult to determine whether an animal is less hyperactive because a tested substance is effectively treating "mania" as intended, or if the animal is simply ill or heavily sedated by the tested drug (5).
Why animal experiments fail
The symptoms of bipolar disorder are complex and deeply rooted in human culture and psychology, which cannot be replicated in animal experiments where only individual symptoms are modeled. The cognitive and emotional aspects of bipolar disorder cannot be mimicked in animals either. Manic episodes, depressive moods, and the associated thought processes are uniquely human. Despite the development of numerous "animal models," none of them can adequately represent bipolar disorder. Most models merely replicate mania or depression by generating symptoms such as hyperactivity or anhedonia. Only a few models consider the cyclical nature of the human condition, where phases of mania and depression alternate (7).
Animal experiments generally assume that the processes involved in a disease are conserved across species. This means that the same genes and proteins play a role in the disease in both humans and animals. However, this assumption cannot account for processes that evolved differently over time (8). Our brain, in particular, differs significantly from that of other animals. For example, the development of the human cerebral cortex is far more complex than in other animals. Therefore, animal experiments cannot reflect the processes involved in human brain development and disease.
Moreover, many disease-causing genes, proteins, or neural circuits involved in mental health disorders are still unknown (5). On what basis can the disease be meaningfully replicated in animals?
Finally, it is generally difficult to assess the validity of animal experiments in the field of psychology. Typically, the usefulness of "animal models" is evaluated as follows: symptoms are induced in the animals, and then it is checked whether common medications for bipolar disorder, such as lithium, alleviate the symptoms. If this is the case, researchers assume that the "model" sufficiently mimics bipolar disorder, allowing new drugs to be tested on it.
However, this approach does not take into account that lithium does not work for all patients. In fact, long-term lithium treatment only prevents manic and depressive episodes in 43% of patients (9). Thus, the "animal model" that appears to be successfully tested due to a positive response to lithium actually corresponds the least to the patients who most urgently need new drugs, as existing treatments do not work for them. This methodological flaw, in addition to the other limitations of animal experiments, makes finding new treatment options even more unlikely.
Researchers are well aware of the lack of "animal models" that actually replicate bipolar disorder and provide insights for human patients. However, as usual, this does not lead to abandoning the animal experimentation system but rather to efforts to further "refine" the models. Different models are also intended to be combined to study individual facets of the disease in separate animal experiments (9).
It is absurd to believe that a complex disease, whose mechanisms are not even fully understood, can be replicated in animal experiments. Only individual aspects or symptoms are mimicked, at the cost of unspeakable suffering for countless animals.
Animal-free research methods
In light of the weaknesses of animal experiments, animal-free methods are gaining increasing importance. These approaches combine technological innovations with human-based models to better understand the mechanisms of bipolar disorder.
In vitro tests
Cell cultures from human tissue offer a more precise way to study the effects of drugs at the cellular level. However, the use of human neurons has been difficult so far, as they are challenging to obtain for ethical reasons. Thanks to induced pluripotent stem cells (iPSCs), neurons from patients can now be generated without exposing the patient to any risk. iPSCs are created by reprogramming somatic cells, such as skin or blood cells. This process involves treating the cells with a cocktail of signaling molecules, which transforms them into pluripotent stem cells capable of differentiating into all cell types, including neurons.
Using neurons derived from iPSCs, the properties of neurons from patients can be compared with those from healthy individuals. Extensive functional analyses have shown how neurons from both groups differ. Additionally, using these neurons, a patient's response to lithium therapy can be predicted with over 92% accuracy (10).
The advantage of such cell cultures is their ease of implementation and the ability to conduct many experiments in parallel. So-called co-cultures, in which other cell types are present alongside neurons, can improve the relevance of cell culture experiments. However, these cultures still lack the architecture and variety of cells and their interactions found in the brain.
Organoids
Mini-brains generated from human stem cells, known as brain organoids, offer the opportunity to study neuronal networks and their dysfunctions in vitro (in the laboratory). They provide detailed insights into the neurobiological foundations of diseases (13). This innovative approach uses three-dimensional cultures of human induced pluripotent stem cells (iPSCs) to generate brain organoids that mimic the early stages of brain development. iPSCs are differentiated into neural progenitor cells under special culture conditions. These cells are then transferred into a three-dimensional culture where they autonomously organize and interact, leading to the formation of brain organoids. These organoids grow and develop over weeks to months, establishing internal neuronal connections and forming various cell types. As a result, brain organoids serve as valuable models for studying human brain development as well as the mechanisms underlying neurological and psychiatric diseases.
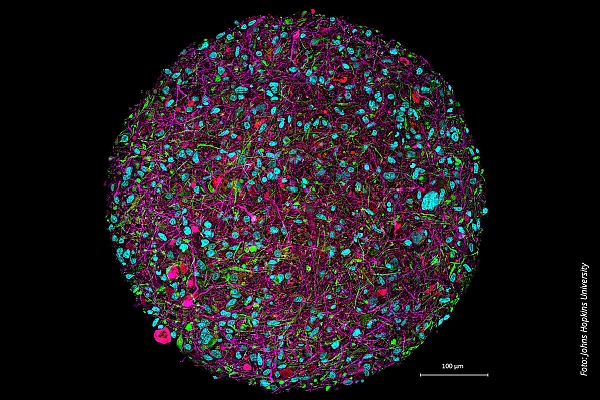
Human brain organoids can be used to study the mechanisms underlying neurological and psychiatric diseases
©Johns Hopkins University
Brain organoids contribute to the understanding of bipolar disorder by enabling researchers to study the molecular and functional changes associated with the condition. In this way, they help to elucidate the underlying mechanisms that potential therapies could target and enable the development and testing of new drugs. Below are some examples of findings about bipolar disorder obtained through the use of brain organoids.
Causal research
Studies using brain organoids derived from iPSCs of patients with bipolar disorder have identified a variety of genes that are differentially regulated compared to healthy controls (11). These genes often overlap with those altered in other psychiatric disorders, such as schizophrenia or major depression, suggesting shared molecular mechanisms (12).
Moreover, organoids from patients with bipolar disorder show smaller sizes, fewer neurons, and less complex neuronal networks compared to control groups (13). This suggests that the developmental processes leading to bipolar disorder involve significant changes in neuronal growth and the networking of neurons. Differences have also been found in relation to ion channels involved in neuronal signaling.
Investigation of pharmacological action
The mechanisms of action of well-known mood stabilizers like lithium are still not fully understood. However, with the help of brain organoids, these mechanisms can now be better studied (13). It has been shown that lithium pretreatment alters calcium signaling and gene expression in the organoids. In organoids derived from patients who respond to lithium, different changes were observed compared to control organoids from patients who do not respond to lithium (14). This contributes to the understanding of lithium’s mechanism of action and helps explain why not all patients benefit from the therapy.
Organoids derived from induced pluripotent stem cells (iPSCs) of patients with psychiatric disorders thus allow researchers to examine specific changes in the cells and assess the effects of new therapeutic approaches.
Personalized therapy
Organoids developed from patient cells retain the genetic and functional characteristics of the patients. This means that the response to drugs in these models can mimic the response in the patient (13). This offers the opportunity to test the efficacy and safety of new medications in a personalized approach before they are used in clinical trials.
Overall, brain organoids represent a promising platform for drug development, as they offer the possibility to study the effects of treatments in a controlled, human, and even patient-specific context.
Organ-on-a-chip systems
Brain-on-a-chip (BoC) systems are artificial models that attempt to replicate the functioning of the human brain in a small, controlled space. These systems are designed to simulate the various cell types and their functions in the brain (15). The different cell or tissue types are cultured in small chambers that are connected by fine channels.
The chips contain multiple cell types to recreate the collaboration between different cells in the brain. This is crucial because the brain's function heavily depends on the interaction between neurons, support cells, and blood vessel cells (endothelial cells). This allows researchers to better understand how normal brain functions occur and how diseases alter the brain. In BoC systems, for example, neurons, microglia (which are involved in the brain's immune response), and endothelial cells (which mimic the blood-brain barrier) can be combined (15).
Thus, BoC systems represent a promising tool for studying psychiatric disorders by replicating the complex biological processes that lead to these conditions in a controlled environment. BoC systems are also being developed in Germany, for example, at the MicroOrganoLab at the Eberhard Karls University of Tübingen (16).
Imaging techniques
Techniques such as fMRI (functional magnetic resonance imaging) and EEG (electroencephalography) allow for the direct analysis of neural activities and networks in the human brain. For example, Roberts et al. studied 97 individuals with a familial risk (at least one first-degree relative with bipolar disorder) and 86 control participants using magnetic resonance imaging, comparing the development of white matter connectivity. They found that in the at-risk individuals, certain brain networks were less well connected (17).
Etiology and prevention
The underlying mechanisms of bipolar disorder are not yet fully understood. One theory suggests that bipolar disorder is linked to a dysfunction in the neurotransmitter system, with various neurotransmitters seemingly involved. A range of environmental factors may also play a role (2). The causes of bipolar disorder are therefore multifactorial, encompassing genetic, neurobiological, and psychosocial factors. Advances in genomics have identified specific risk genes.
Due to the complex and still insufficiently understood mechanisms of onset, prevention is difficult. Here, animal-free methods such as brain organoids could help, for example, to better understand the effect of environmental influences on brain development and thus identify preventable risk factors.
Conclusion
Research on bipolar disorder faces significant challenges. Animal experiments, which were long considered indispensable, are increasingly proving to be unproductive. Additionally, they are exceedingly cruel, and the methods used to induce symptoms through repeated stress over extended periods would be considered torture if applied to humans.
Advances in animal-free methods, such as organoids and organ-on-a-chip systems, can not only address ethical concerns but also provide more precise and effective treatment options.
28.01.2025
Dr. rer. nat. Johanna Walter
References
- Bipolar disorder (in German), Neurologen und Psychiater im Netz, Das Informationsportal zur psychischen Gesundheit und Nervenerkrankungen
- Valvassori S.S. et al. Contributions of animal models to the study of mood disorders. Revista Brasileira de Psiquiatria 2013; 35(suppl 2):S121–S131
- Gessa G.L. et al. Sleep deprivation in the rat: an animal model of mania. European Neuropsychopharmacology 1995; 5:89–93
- Li X. et al. A novel murine model of mania. Molecular Psychiatry 2023; 28(7):3044–3054
- Logan R.W. et al. Animal models of bipolar mania: The past, present and future. Neuroscience 2016; 321:163–188
- Sahin Z. Assessment of commonly used tests in experimental depression studies according to behavioral patterns of rodents. Medical Review 2023; 3(6):526–531
- Beyer D.K.E. et al. Animal models for bipolar disorder: from bedside to the cage. International Journal of Bipolar Disorders 2017; 5(1):35
- Cheng K. et al. Comparison of model systems for emulating human tissue and physiology. Psychiatric Research 2024; doi: 10.20944/preprints202408.0270.v1
- Maj M. et al. Long-term outcome of lithium prophylaxis in bipolar disorder: A 5-year prospective study of 402 patients at a lithium clinic. American Journal of Psychiatry 1998; 155(1):30–35
- Stern S. et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Molecular Psychiatry 2018; 23(6):1453–1465
- Kim K.H. et al. Transcriptomic analysis of induced pluripotent stem cells derived from patients with bipolar disorder from an old order Amish pedigree. PLOS ONE 2015; 10(11):e0142693
- Gordovez F.J.A. et al. The genetics of bipolar disorder. Molecular Psychiatry 2020; 25(3):544–559
- Osete J.R. et al. Transcriptional and functional effects of lithium in bipolar disorder iPSC-derived cortical spheroids. Molecular Psychiatry 2023; 28(7):3033–3043
- Chen H.M. et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Translational Psychiatry 2014; 4(3):e375
- Amirifar L. et al. Brain-on-a-chip: Recent advances in design and techniques for microfluidic models of the brain in health and disease. Biomaterials 2022; 285:121531
- Brain-on-Chip, MicroOrganoLab
- Roberts G. et al. Longitudinal changes in structural connectivity in young people at high genetic risk for bipolar disorder. American Journal of Psychiatry 2022; 179(5):350–361
