Mini brains: innovative, human-relevant brain research
Human “mini brains” are small 3D structures that mimic the structural and functional properties of the brain. Thanks to these advanced research systems, many questions that have been explored unsuccessfully for decades in various animal experiments have been answered. Mini brains have the potential to allow scientists to finally make progress in treating some of the most serious diseases to date, such as Alzheimer's disease, Parkinson's disease, autism, various viral infections, and cancer.
The human brain - a mysterious universe
The brain is the most complex organ of the human body, consisting of billions of cells of different types having various functions. They work together in a spatially and temporally coordinated manner to provide and regulate higher cognitive and motor functions such as the control of organs, body temperature, the sleep-wake cycle, movements, emotions, and behavior. Human brain development begins in the third week of pregnancy and relies on several tightly regulated cellular and molecular sequential processes. Many questions regarding these processes are still open. For example, it is not yet known how the great diversity of cell types in the human brain develops at the right time, in the right place, and in the right quantities. How do the brains of individual people differ? What goes wrong when brain cells do not develop normally? And how do neurological diseases affect the organization and functions of the brain?
Animals are not a good "model" for the human brain
An important reason for the lack of success in answering these questions so far is based on the fact that very often different animal species are used as "models" for the study of human brain processes and diseases. It is well known that there are crucial differences between humans and animals in the structure, development, organization, and functions of the brain. Furthermore, severe neurological and neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and autism occur naturally only in humans. Numerous drugs and therapies that have proven safe and effective in countless animal studies have failed in clinical trials with human patients because they did not achieve the desired outcomes and sometimes even caused severe side effects. More than 400 Alzheimer's drugs, for example, have been successfully tested in animals; none of which have worked in humans (1). Some, such as the drug verubecestat, which was highly praised in animal preclinical studies on mice, rats, rabbits, and monkeys, have even accelerated and worsened disease symptoms such as dementia in humans (2). Effective therapies for Alzheimer's disease, autism, bipolar disorder, and many other neurological and cognitive disorders do not currently exist or the few that are used cannot completely cure the diseases. To better understand the causes of these diseases and develop appropriate drugs and therapies for them, a modern, human-based research approach has been established - the so-called mini brains.
Mini brains: small brains with big potential
Mini brains are three-dimensional (3D) structures the size of a few millimeters, made of human cells. They mimic many anatomical and functional features of the human brain. Compared to traditional cell cultures, which consist of a single uniform 2D layer of cells on the bottom of a petri dish, mini brains can have multiple layers of different, interconnected cell types positioned in a 3D spatial environment very similar to the brain microenvironment in the human body. Thus, they offer scientists the opportunity to study parts and functions of the brain that were previously inaccessible.
Mini brains are generated from stem cells like the so-called induced pluripotent stem cells (iPSCs). Brain tissue is not necessary. A few blood, skin, or hair root cells, for example, can be taken from healthy and diseased people and reprogrammed into iPSCs. By adding certain growth factors, the iPSCs develop into mini brains, which - depending on the cell donor - are healthy or reflect the donor's disease. On average, it takes about 15 weeks to generate a mini brain that mimics the brain of a five-month-old human fetus (3). Mini brains can be stored frozen and thawed as needed so they do not have to be generated every time they are needed. Some mini brains can be used continuously for over 800 days, making them particularly well suited for long-term studies on developmental processes and neurological diseases (4).
A major advantage of mini brains is that they can be generated in large numbers with the same characteristics. This makes them particularly suitable for screening using automated high-throughput platforms of active substances and drugs (5).
Different Mini Brain types for each research question
Depending on the structure, properties, and manufacturing strategies, there are different types of mini brains.
Brain organoids are grown in special bioreactors. They develop spontaneously and contain many different cell types organized into brain regions such as forebrain, midbrain, hindbrain, and retina similar to the human brain. Brain organoids are very diverse but can vary widely based on their spontaneous self-organization (5).
The so-called brain spheroids are small spherical structures that contain fewer cell types than the organoids. Their production involves the use of several growth factors specific to certain brain regions. Therefore, spheroids often represent a specific brain region such as the cerebral cortex, hippocampus, or midbrain. Compared to brain organoids, spheroids are less developed and diverse, but their production is simpler and more uniform (5).
Sometimes, two or more separately grown brain-region-specific spheroids are fused in a controlled manner to form brain assembloids with multiple distinct areas. For example, fused spheroids of the dorsal (upper) and ventral (lower) forebrain form an assembloid with two distinct but interconnected areas whose cells establish functional connections with each other (5).
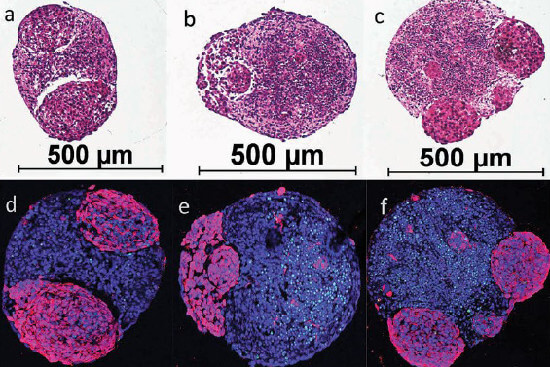
Human mini brains developing brain tumors. Microscopic images of the stained cell cultures (brain spheroid), grown from human iPSCs (induced pluripotent stem cells) in a cell culture plate. Below (d-f), the healthy brain tissue is blue and the tumors are purple. The diameter of the mini brains is approximately 0.5 mm. Image source: Plummer et al - SciRep 2019 (6).
A variety of applications in medicine and biology
Mini brains provide scientists with the ability to study brain processes, diseases, and therapies in a complex, human-relevant system without invasive surgery on patients. Furthermore, patient-specific mini brains from individual humans can be derived via iPSCs and treated with different drugs, facilitating a huge step toward personalized medicine - the medicine of the future.
Mini brains are well suited for the analysis of developmental disorders because they can reliably mimic various prenatal brain malformations and their associated cellular and molecular disorders (5). A study using human mini brains allowed the identification and investigation of the genetic and environmental factors that lead to deformity and undersized brain size in microcephaly and Zika virus infection (7). Interestingly, the study also showed that the disease-specific changes in human mini brains do not occur in mini brains derived from mouse cells, which is one of the myriad evidences of how different human and animal brains are. This also highlights how important it is to explore human-based, instead of animal-based, "models". Another study based on mini brains identified several substances that may be useful against the Zika virus (8). Several research groups are using human mini brains to study the mechanisms of other viruses such as dengue virus, HIV, and John Cunningham virus. SARS-CoV-2, the cause of the coronavirus pandemic, can also infect mini brains, and studying the mechanisms of infection may provide important insights into the neurological symptoms that occur in many COVID-19 patients (9).
Common psychiatric disorders such as depression, autism, schizophrenia, and bipolar disorder have, until recently been virtually impossible to study at the molecular level. The success rate of drug development in this area is appallingly poor – a total of only 33 drugs have been approved for psychiatric disorders since 1975 (10). The difficulty in studying these disorders lies in the lack of appropriate models. Standard animal studies cannot reflect the complexity and symptoms of human mental disorders at all. Analyses of human brains are usually only possible in the last phase of illness during autopsies, and even individual patients differ greatly from one another. The ability to derive personalized mini brains from individual patients and use them for screening and evaluating potential therapies has great potential for improving drug development success in psychopharmacology (11).
In recent years, it has become possible to use mini brains to study many neurodegenerative diseases. One publication presents a high-throughput screening of human midbrain organoids for the study of Parkinson's disease and other neurodegenerative disorders (12). Other serious neurodegenerative diseases that are affecting increasing numbers of people and for which effective therapies are currently lacking include Alzheimer's disease, amyotrophic lateral sclerosis (ALS), and Huntington's disease. Just as for psychiatric disorders, mini brains offer a good approach for analyzing these diseases and developing effective drugs (13). Human mini brains, for example, are suitable for research on Alzheimer's disease because they reflect many of the complex molecular features of the disease (13). In comparison, the commonly used "mouse models" do not have all of the human-specific brain cell types and areas that play a role in AD, which is almost certainly the main reason for the disastrous results of these animal studies for drug development.
Malignant cancers and brain tumors can also be studied using mini brains. In one study, for example, it was shown that two different cancer drugs attack the tumors and inhibit their growth, while the healthy brain cells remain largely unharmed (6). In principle, this test system works with mini brains from any donor and with different brain tumor cells.
Because the brain controls the functions of all other organs, these are often affected by various neuronal disorders. To study the interactions between brain, nerves, and other organs, mini brains can be connected to other human organoids on a so-called organ-on-a-chip (OoC) or multi-organ chip (MOC). A MOC is a system that can link up to 10 human mini-organs via an artificial vascular network and, if necessary, a urinary system. In this way, the mini-organs exchange metabolites, just like the organs in the body do. For example, a blood-brain barrier chip can accurately predict whether a drug can cross over from the blood into the brain (14). Chips made from individuals with neurological disorders provide information about the location of disruptions in the blood-brain barrier. Another study showed that brain-spinal-cord assembloids linked to mini-muscles are able to produce muscle twitches after stimulation of the mini brains (15).
Mini brains can be used not only to study various diseases, but also to increase our understanding of human evolution. The brain, particularly the cerebral cortex, has evolved at a particularly high rate in humans compared to other animal species. A better understanding of this species-dependent difference will help us to understand the mechanisms of evolutionary brain development more precisely. Because only skin or hair cells are necessary to generate mini brains, they open up new possibilities for comparative studies of the brain across species. For example, a comparison between iPSC-derived mini brains from humans, chimpanzees, and macaques revealed differences in brain cell development that lead to different outcomes, which may explain the differences in cortex size between primate species (5,16). A similar study showed that basic neurological and genetic developmental processes are very different in monkeys and humans (17).
This is further proof that monkeys are not suitable for studying the human brain and that future biomedical research must use modern human-based methods that are already available today.
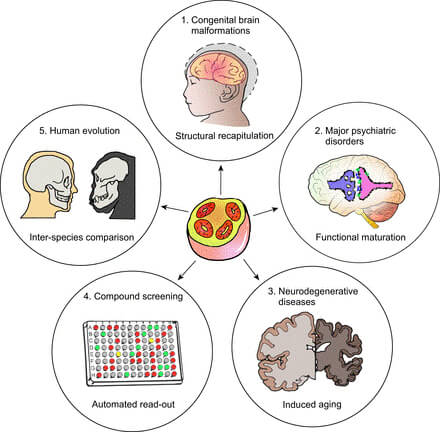
Figure: Some examples of the potential applications of human mini brains (center): 1. study of developmental processes and brain malformations 2. analysis of psychiatric diseases 3. study of neurodegenerative diseases 4. development and testing of drugs 5. better understanding of human evolution. Source: Qian et al. 2019 (5)
Opportunities for improvement
Although mini brains already offer good research opportunities for many questions that have been difficult to answer, they also have certain limitations and potential for improvement. First of all, the neuronal cells in the mini brains are usually not as mature as those in the adult brain and are more similar to the cells of the fetal brain. The generation of the more complex brain organoids is often variable, making it difficult to compare studies. Furthermore, most mini brains currently lack blood vessels, which compromises their oxygen supply. Because of this, the mini brains die when they reach a certain size because oxygen can no longer diffuse from the environment to their interior. For this reason, the mini brains are sometimes implanted into living animals, such as mice, whose blood vessels grow into the mini brains and supply them with oxygen. However, this procedure cannot be condoned due to ethical and scientific reasons, since chimeras made from human tissue and an animal organism cannot mirror the processes that naturally occur in the human body. In addition, most mini brains do not possess immune cells, which are important for defense reactions and for reactions to test substances.
Many research groups are already working hard to overcome these limitations and improve the mini brain models. In recent years, these systems have become much more complex. It is already possible to generate and analyze multiple brain areas simultaneously in the same mini brains. There are research groups that have succeeded in growing mini brains with human blood vessels, immune cells, and connective tissues (18). The rapid development of mini brain models to date and their great potential for biomedical research and drug development show that the current problems can soon be solved. However, for this to happen, this innovative research field needs adequate funding. Currently, more than 99% of public funding for biomedical research in Germany is wasted on misleading animal experiments, while less than 1% goes to modern, human-based methods. In the future, these promising, human-relevant technologies must be much better funded if we want to better understand human brain processes and effectively treat diseases.
Can mini brains think?
From an ethical perspective, many people ask whether human mini brains could think and develop some sort of consciousness. While mini brains mimic many brain processes, the scientific community agrees that they do not have these capabilities. Mini brains do not have thoughts, feelings, or sensations and therefore can be safely used for experiments.
Conclusion
Human mini brains are small 3D structures that mimic the anatomical and functional properties of the human brain and have great potential for studying the processes of the healthy and diseased brain. Many important questions about brain development, neurodegenerative, psychiatric, and oncological diseases, and human evolution have already been answered thanks to these systems. Experts expect a rapid development and improvement of the mini brains in the near future to better reflect the more complex biological properties of the brain. Many innovative studies with human mini brains are already summarized in our NAT database for non-animal research methods (www.nat-database.org, NAT = Non-Animal Technologies) (19).
01 February 2021
Dr. rer. nat. Dilyana Filipova
References
- Bennett DA. Lack of benefit with idalopirdine for Alzheimer disease: Another therapeutic failure in a complex disease process. 2018; 319: 123–5
- Egan MF et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. New England Journal of Medicine. 2019; 380: 1408–20
- Yadav A et al. Brain organoids: tiny mirrors of human neurodevelopment and neurological disorders. Neuroscientist. 2020; 1073858420943192
- Marx V. Reality check for organoids in neuroscience. Nature Methods. 2020; 17: 961–4
- Qian X et al. Brain organoids: advances, applications and challenges. Development. 2019; 146 (8)
- Plummer S et al. A Human iPSC-derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Scientific Reports 2019; 9: 1407
- Li Y et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell. 2017; 20: 385-396.e3
- Watanabe M et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Reports. 2017; 21: 517–32
- Bullen CK et al. Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. 2020; doi: 10.14573/altex.2006111
- van der Doef TF et al. New approaches in psychiatric drug development. European Neuropsychopharmacology. 2018; 28: 983–93
- Rossetti AC et al. Drug discovery in psychopharmacology: from 2D models to cerebral organoids. Dialogues in Clinical Neuroscience. 2019; 21: 203–24
- Renner H et al. A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. eLife. 2020; 9: e52904
- Venkataraman L et al. Modeling neurodegenerative diseases with cerebral organoids and other three-dimensional culture systems: focus on Alzheimer’s disease. Stem Cell Reviews and Reports. 2020; doi: 10.1007/s12015-020-10068-9
- Vatine GD et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019; 24: 995-1005.e6
- Andersen J et al. Generation of functional human 3D cortico-motor assembloids. Cell. 2020; 183: 1913-1929.e26
- Otani T et al. 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell. 2016; 18: 467–80
- Kanton S et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature. 2019; 574: 418–22
- Wörsdörfer P et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Scientific Reports. 2019; 9: 15663
- NAT Database, www.nat-database.org