Multiple sclerosis: animal-based and animal-free research
Multiple sclerosis, MS, encephalomyelitis disseminata or G35 - all these terms describe a chronic inflammatory disease of the central nervous system in which the nerve fibres are damaged. The disease is multifactorial, and the exact causes remain unclear, which means that so far, no drugs have shown a resounding therapeutic success. A crucial hindrance on this path are the “animal models” used. The ones that are available only show similarities to MS and reproduce only some of the stages of MS. Human studies, on the other hand, can really shed light on the matter.
In Germany, around 250,000 people suffer from the degenerative nerve disease; 2.8 million are affected worldwide (1). The disease onset is usually between the ages of 20 and 40, but there are also cases in which symptoms appear in childhood. Women are affected about twice as often as men. Typical symptoms are signs of paralysis and spasticity, especially in the legs, as well as visual disturbances, sensory disturbances on the skin, swallowing and speech problems, all of which can be associated with pain. Symptoms often come in flares, with one or more symptoms appearing over a period of at least 24 hours to several weeks.
Especially at the beginning, they can recede completely. Sometimes, the recess is only partial (Relapsing-Remitting MS = RRMS). As the disease progresses, remission is usually incomplete, so that the symptoms or the severity of the symptoms add up and thus gradually impair the lives of those affected. The clinical course displays a gradual steady deterioration with possible relapses - this is called Secondary Progressive MS (SPMS). In about 10% of those affected, the condition slowly deteriorates from the start without clearly distinguishable relapses; this is referred to as Primary Progressive MS (PPMS) (1).
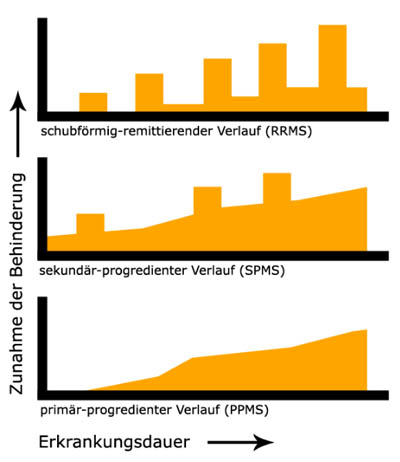
Clinical courses of multiple sclerosis. Wikipedia
Since the disease displays such varied symptoms and courses, a prognosis after diagnosis is hard. On average, half of those affected are dependent on a walking aid after 15 years, and after 25 years, a third is no longer able to walk (2).
The decisive factor for the disease mechanism is the destruction of the myelin, the insulating layer of the axons of the neurons in the brain and spinal cord. This is formed by oligodendrocytes in the central nervous system and by Schwann cells in the peripheral nervous system. The so-called myelin sheaths, which encase the nerve processes, are a crucial part of enabling faster signal transmission. If the layer is partially or completely destroyed, nerve signals can only be transported slowly, insufficiently, or not at all - the result are precisely those muscle activity and sensitivity impairments described above.
The reason for the destruction of this important substance is a misguided immune system: it attacks the myelin of patients and damages it. This happens at several (>> multiple) locations in the brain and spinal cord. Particularly in the early stages, remyelination can still occur. Additionally, scarring (>> sclerosis) occurs in certain patients as the disease progresses. Sclerosis spatially occurs in clusters, which are visible on MRI once they have reached a certain size (3).
MS can be classified as an autoimmune disease, although the causes that trigger the immune system in this way are still not exactly known. They are probably multifactorial, ranging from genetic to environmental aspects, and result in a lymphocyte-mediated autoimmune inflammatory reaction directed against certain proteins in the body's own myelin sheaths.
Animal experiments in MS
Most experiments in this area are conducted in mice, but also on rats, macaques, or common marmosets. Zebrafish have been used more frequently lately. Monkeys are used particularly for the very painful toxicity testing of potential drug candidates (4).
Some examples:
The 70 male and female mice that were used in an experiment derive from an inbred line that is particularly susceptible to sex-dependent experimental autoimmune encephalomyelitis (EAE). This is an artificially induced inflammatory disease of the central nervous system that is used as a "model" in multiple sclerosis research. In order to trigger EAE in the mice, they are injected with a solution of a special protein and inactivated pathogens. In addition, a bacterial poison is injected twice into the abdominal cavity. Then, the development of the symptoms of EAE is observed. It ranges from signs of paralysis of the tail towards the hind legs to the front legs before the animals finally die. Mice that are paraplegic or have paralyzed forelimbs are killed in undisclosed ways. In this particular experiment, this applies to three animals. At the peak of the disease, diseased and healthy mice are fixed in a special holder and anesthetized with a gaseous anaesthetic. The animal’s heads are exposed to vibrations and examined using an imaging method. After that, the mice are killed with an overdose of anaesthetic and the blood in their bodies is replaced with a preservative solution. The brains are removed and examined. The aim is to find out whether the brains of male and female mice soften to different degrees as a result of the disease (5).

Most MS experiments are conducted in mice. (Image: Vasily Koval Adobestock)
In another experiment, mice, which are highly social animals, are housed individually in cages. By feeding them a chemical, the protective myelin is degraded. The animals are sacrificed on days 7, 25, or 45 after the beginning of the feeding to analyze the extent of brain damage.
A second group of mice is treated with lysolecithin, which specifically damages certain regions of the brain. To do this, the mice are anaesthetized, their heads are fixed in a stereotactic apparatus, a hole is cut in the skull bone, and the substance is injected with a needle at specific points in the brain, where it destroys the myelin. After 7 days, the animals are sacrificed by sedation with isoflurane, an inhalation anaesthetic that hardly relieves pain, then their chest is cut open, and a preservative solution is exchanged for the blood.
In another study, mice are anaesthetized and electrodes are surgically implanted in the brain. 7 to 10 days later, nerve signals are recorded on the unanaesthetized animals via the electrodes before, during, and after exposure to sound stimuli. After the tests, the animals are killed by an injected substance. Furthermore, the mice are subjected to a so-called fear conditioning test. One mouse at a time is enclosed in a small chamber and sounds are played, some of which are combined with electric shocks (administered through the floor of the chamber) to train fearful behaviour. In one day, each mouse is subjected to auditory electric shock treatment six times. After 24 hours, it is checked whether the animal reacts with rigid fear to the isolated sound not linked to an electric shock. On another day, the procedure is repeated in a similar form with the same animals.
The consequences of myelin destruction include hearing and vision disorders, as well as impaired perception and motor functions. Reversing the process of myelin destruction is a therapeutic approach that is already being studied in human subjects - yet agonizing animal testing is still conducted in this area (6).
The explanation for a series of experiments with 10 marmosets is the poor correlation between treatment success in "animal models" for multiple sclerosis and application in patients. In marmosets, the disease is variable, dramatic, and rapid with severe symptoms of paralysis, and death before treatment can be carried out. Here the authors present a new "animal model" in which the monkeys do not die that quickly.
Under anaesthesia, six monkeys are given four injections of a mixture of recombinant (genetically modified) rat nerve sheath protein and an irritating mixture of mineral oil and inactivated tuberculosis bacteria subcutaneously on the shoulders and hips. This alters the body's defence system. 70 days later, under anaesthesia, the animals’ heads are clamped in a stereotactic frame and a hole is drilled in the cranial bone. The brain tissue is pricked with a cannula and certain inflammatory factors are injected. This causes the body's immune system to attack and destroy the myelin sheaths in the area of the injection, which is said to resemble symptoms of human MS.
The animals suffer from paralysis of the legs. During the course of the experiment, three blood samples are taken and the animals' heads are scanned three times using an imaging method. The animals are anaesthetized for this. Three monkeys are treated in the same way, except that, before the first injection, these animals will be treated with a multiple sclerosis drug that is already in Phase III clinical trials in humans. 90 days after the first injection, all monkeys are sacrificed by an anaesthetic overdose and the brains are examined (7).

Marmosets are often used in MS research. (Image: amphawan Adobestock)
Why animal experiments in MS research fail
Basically, the MS animal experiments are based on the same problematic principle as all animal experiments: young, healthy animals are artificially manipulated to show symptoms of the disease similar to those of humans affected by the disease. In MS in particular, the authors, who defend the available "animal models", have to admit that all available models show only certain, isolated human symptoms of MS. None shows the entity of symptoms of the disease in humans (4).
The so-called EAE models are often used, i.e. an experimental autoimmune encephalomyelitis is induced. For this purpose, rodents such as mice or rats are injected with an antigen aligned with an active enhancer, usually the so-called Freund's adjuvant, an emulsion of water and paraffin oil which may still contain inactivated tuberculosis bacteria. This results in an inflammatory reaction and thus damage of the spinal cord. It sometimes also leads to lesions of the cerebellum and the optic nerve. However, the EAE lesions are mainly characterized by degeneration, i.e. breakdown of the axons, and less by demyelination - a fundamental difference from the damage found in MS patients. The EAE model is also unable to recapitulate the demyelination of the forebrain and gray matter as well as other MS characteristics of the white matter (8).
Only mice can be infected with Theiler's murine encephalomyelitis virus (TMEV), causing them to show similar symptoms to EAE rodents. In mice, the axons degenerate first and then the myelin - exactly opposite of what is observed in humans.
Another method is the addition of the chemical cuprizone to the chow, which is mostly done in mice but also in rats. The substance kills oligodendrocytes in the brain in a way that is not yet understood, leading to paralysis over time. However, different mouse strains also show different lesion patterns in the brain. The sex of the animals also has an influence. Mega-mitochondria form in the liver, a sign of a disturbed metabolism that may be responsible for the death of the oligodendrocytes (4). However, the liver is a particularly important organ when it comes to the effectiveness and tolerability of drugs. Considering the generally low transferability of results from drug research this probably has additional questionable effects. In addition, this paralysis is reversed as soon as cuprizone is no longer administered (8). Remyelination also occurs in humans in the early stages of the disease, but for completely different reasons. Thus, the decisive aspect, namely which mechanism triggers a new disease phase, cannot be examined in the "animal model". The prevention of a new phase would be an extremely important point for therapy, though.
In addition to the mice that are frequently used, zebrafish and primates such as marmosets are also used in various experiments, as described above. But keep in mind: even a combination of different (often genetically modified) animal strains, the often tried and highly praised humanized mice and antigens, can only reproduce parts of the human symptoms in an alien system.
In recent years, some MS drugs have been newly approved. The general success rate for MS drugs is 27%, which is higher than the average for all drug classes, which is only 8% (9,10). And still, there are only therapies to mitigate flare-ups and reduce frequency to date., There are no treatments available for the slow and progressive worsening of underlying symptoms. In addition, many of the drugs approved in recent years are based on older ones. Some of them are improved developments of substances that have been on the market for some time, so it is quite probable that they will work in both animals and humans, which is an explanation for the rather high quota.
Every (MS) patient is understandingly happy and hopeful about every new type of therapy, but immunotherapies in particular are associated with severe side effects. In principle, most of these drugs work by suppressing the body's own immune system - you don't have to be a physician to suspect that this may have serious disadvantages.
Until interferons were approved, flare-ups were treated with high-dose cortisone. However, not all MS patients actually benefit from this therapy: only 9 out of 100 showed no further increase in impairment and only 15 out of 100 remained relapse-free. Other drugs are partially effective in a larger number of patients, but more than 40 out of 100 do not benefit in any case, while side effects are very common (11).
The monoclonal antibodies, which have been used for several years, are very effective - but also have serious side effects. Daclizumab, for example, was withdrawn from the market in 2018 because it caused acute liver failure and encephalitis (12). Although Alemtuzumab is on the market, it is classified as a reserve drug and is rarely prescribed anymore, as it led to another autoimmune disease in 30% of those treated, and 90% suffered side effects such as headaches, fever, and allergies. Furthermore, there is an increased risk of infection. It is particularly concerning that, in some cases, side effects may persist even after stopping some MS medications (13).
Against this background, it is not surprising that a comprehensive information brochure from the Hamburg-Eppendorf University Hospital states: "[...] The price for a high level of effectiveness in preventing relapses are sometimes equally high side effects. Somewhat sobering: the long-term benefit remains unclear. A direct effect on the degenerative part of the disease, in which nerve cell structures are destroyed and which is probably responsible for a large part of the permanent impairment, has not yet been shown. […]” (11).
There are certainly many reasons for this, but sticking to artificial, foreign-species systems that cannot reflect the human situation and which are nevertheless a prerequisite for market approval is a major and underestimated shortcoming.
The better choice: alternative methods
There are many factors that can possibly play a role in the development, outbreak, and course of the disease. Population studies have so far shed the most light in understanding MS.
A link to Vitamin D deficiency was stated as early as 2006 (14). Furthermore, a recent study involving around 20,000 subjects, half of them diagnosed with MS, concluded that at least 13% of MS cases could be prevented by not smoking (15).
The gut, whose diverse influence on many different diseases has only recently come into focus, seems to play a role in MS, since both the intestinal flora (16) and the mycobiome (17) show certain changes in MS patients. This can affect the progression of the disease, but also the response to therapies.
Mathematical models also use data from population studies. In a study that evaluated patient data from a time span of 20 years, it was shown that despite the differences in the various MS subtypes, there appears to be a common pathogenesis - an important finding for further therapy development (18).
The biggest stir was probably created by a publication in 2022 in which the data of 10 million people was evaluated. Such a large amount of data could be gathered due to a collaboration with the US military, making data from 1993 to 2013 available. Of 801 people who developed MS while serving in the military, only one person was free of Epstein-Barr virus (EBV) infection. The bottom line is that it is very likely that infection with this virus is the main cause of MS. Although infection with this human herpesvirus is very common and the assumption that EBV infection is associated with MS has existed for years, such a clear connection had not been proven before (19).
Thus, an important root regarding the cause of MS seems to be uncovered. EBV is a human pathogenic virus, so that the development and progression of the disease and potential therapies in human systems should or must be examined from this point of view in particular.
As in almost every other area, cell cultures are also used in MS research to study fundamental mechanisms at a cellular level. Cells obtained from MS patients are particularly suitable here, as they have preserved the disease-specific information. Cells from healthy donors can be used for comparison. In various studies, the involvement of certain proteins such as receptors, but also the influence of neurotransmitters on the disease mechanism could be examined and biomarkers identified (20–22).
Particular attention is paid to the oligodendrocytes, the myelin-producing cells. By reprogramming already differentiated body cells (e.g. a hair root cell), so-called iPSCs (= induced Pluripotent Stem Cells) can be obtained from MS patients. These can then be further differentiated into any cells - e.g. first into precursor oligodendrocytes, which then develop into mature oligodendrocytes. In this way, an oligodendrocyte cell line can be created.
If these cells are obtained from MS patients who are in a stable phase of MS, they usually do not show any particular abnormalities. However, when an endogenous interferon, which is known to play a negative role in MS, is added to the culture, oligodendrocyte differentiation is found to be highly reduced. If the precursor oligodendrocytes cannot differentiate into mature oligodendrocytes, the formation of new myelin is also impaired. In MS patients, reduced myelination leads to reduced nerve conduction, which then causes paralysis and reduced sensation. Such a cell culture can therefore be used to add different combinations of inflammatory substances and to find out which influence oligodendrocyte differentiation and thus myelination and how strongly. This knowledge may be important in understanding how diverse and effective therapies can be in different inflammatory cell environments (23).
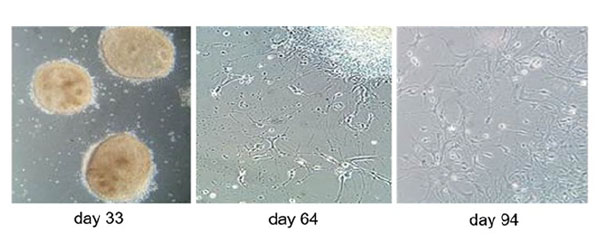
Differentiation stages of iPSC-derived oligodendrocytes. (Image: Morales Pantoja et al. PLoS One 2020)
In contrast to the classic 2D cell cultures, there are also three-dimensional models: neurospheres are clusters of nerve cells floating freely in a solution, the progenitor cells of which can be used for research purposes in particular. This method, in which the spheres also fuse, has been further developed to include multiple cell types (24).
Using a 3D brain organoid model, also called BrainSphere, generated from human iPSCs, the myelination and demyelination processes in the axons, which are crucial in this disease, can be examined. A myelin damaging substance, which served as a positive control, also attacked the myelin in this model, while ibuprofen (negative control) had no effect. The model is therefore suitable for testing potentially harmful substances that can attack the myelin (25).
Organoids possess the advantage that they are more complex than classic 2D cell models and can better represent the human situation. The 3D cell models can either be derived from primary cells or produced using iPSC technology. For the former, a biopsy must be taken from the respective tissue - if this is medically required anyway, some cells can be diverted. However, this is often not the case and no longer necessary since iPSC technology is available. This is particularly useful when researching diseases such as MS, since it is mainly the brain that is affected and brain cells from patients are often not available.
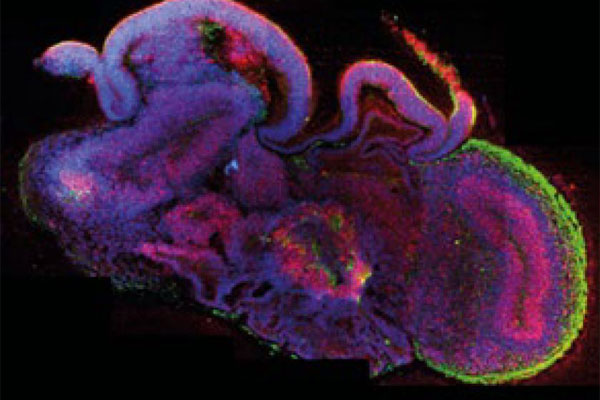
Brain organoid. (Image: Lancaster, M.A. Nature 2013; 501: 373-379)
Patient-specific organoids can be generated (in addition to control organoids from healthy donors) from all three forms of MS: relapsing-remitting, secondary progressive, and primary progressive MS. In this way, the (possible) differences between the various forms of progression can be examined. Using these organoids, it could be determined e.g. that in the MS forms, but especially in PPMS, the differentiation of the oligodendrocytes is reduced compared to the healthy organoids, i.e. the cells that are responsible for the construction of the myelin. In addition, a lower expression of a cell cycle inhibitor was found, so that a possible therapeutic approach is displayed here (26).
3D bioprinting has been spreading for a few years and also offers great potential for MS research. The technology is currently used primarily to precisely map the typical MS damage in the brains of patients. This method is more precise and accurate than MRI scans, and a better understanding of the lesions ultimately aids in understanding disease progression and therefore in improving therapies.

3D print of the brain of an MS patient. (Image: Jose Santoyo, Stratasys)
In addition to (basic) research, the human-based methods also offer the possibility of immediate patient-specific therapy: A new research project at the University of Aarhus (Denmark) aims to create new artificial, myelinated nerve fibers by employing electrospun fibers (27). It may take some time before this is actually applicated in the clinic, but compared to how rapidly human-based, modern methods have developed in recent years despite completely insufficient funding, it could become reality faster than anyone could hope.
13 April 2023
Dipl. Biol. Julia Radzwill
References
- Deutsche Multiple Sklerose Gesellschaft Bundesverband e.V. Website
- DocCheck Flexikon. Multiple Sklerose
- Pschyrembel Online. Multiple Sklerose
- Burrows DJ et al. Animal models of multiple sclerosis: From rodents to zebrafish. Multiple Sclerosis Journal 2019; 25(3):306 –324
- Batzdorf C.S. et al. Sexual dimorphism in extracellular matrix composition and viscoelasticity of the healthy and inflamed mouse brain. Biology 2022; 11(2):230
- Cerina M. et al. The quality of cortical network function recovery depends on localization and degree of axonal demyelination. Brain, Behavior, and Immunity 2017; 59:103–117
- Stassart R.M. et al. A new targeted model of experimental autoimmune encephalomyelitis in the common marmoset. Brain Pathology 2016; 26(4):452–464
- Kipp M. et al. Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathology 2017; 27(2):123–137
- De Gasperis-Brigante C.D. et al. Reducing clinical trial risk in multiple sclerosis. Multiple Sclerosis and Related Disorders 2016; 5:81–88
- Thomas D et al Clinical Development Success Rates, 2021.
- UKE Hamburg. Immuntherapien der schubförmigen Multiplen Sklerose 2021. 2021
- Ärzte gegen Tierversuche e.V. Schwerwiegende Nebenwirkungen bei Multiple-Sklerose-Medikament, 6.3.2018
- Neurologie Essen. Aktuelle Therapiemöglichkeiten der schubförmigen MS, 2021
- Munger K.L. et al. Serum 25-Hydroxyvitamin D levels and risk of Multiple Sclerosis. JAMA 2006; 296(23):2832–2838
- Manouchehrinia A. et al. Smoking attributable risk in Multiple Sclerosis. Frontiers in Immunology 2022; 13
- iMSMS Consortium. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell 2022; 185(19):3467-3486
- Shah S. et al. Alterations of the gut mycobiome in patients with MS. eBioMedicine 2021; 71
- Kotelnikova E. et al. Dynamics and heterogeneity of brain damage in multiple sclerosis. PLoS Computational Biology 2017; 13(10):e1005757
- Bjoernevik K et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375(6578):296-301
- Faigle W. et al. Brain citrullination patterns and T Cell reactivity of cerebrospinal fluid-derived CD4+ T cells in Multiple Sclerosis. Frontiers in Immunology 2019; 10:540
- Etesam Z. et al. Different expressions of specific transcription factors of Th1 (T-bet) and Th2 cells (GATA-3) by peripheral blood mononuclear cells from patients with Multiple Sclerosis. Basic and Clinical Neuroscience 2018; 9(6):458–469
- Clottu A.S. et al. EBI2 expression and function: Robust in memory lymphocytes and increased by Natalizumab in Multiple Sclerosis. Cell Reports 2017; 18(1):213–224
- Pantoja I.E.M. et al. iPSCs from people with MS can differentiate into oligodendrocytes in a homeostatic but not an inflammatory milieu. PLOS ONE 2020; 15(6):e0233980
- Watanabe F. et al. Generation of Neurosphere-Derived Organoid-like-Aggregates (NEDAS) from neural stem cells. Current Protocols 2021; 1(2):e15
- Chesnut M. et al. Human iPSC-derived model to study myelin disruption. International Journal of Molecular Sciences 2021; 22(17):9473
- Daviaud N. et al. Cerebral organoids in primary progressive multiple sclerosis reveal stem cell and oligodendrocyte differentiation defect. Biology Open 2023; 12(3):bio059845
- Drug Target Review Drug Target Review: New treatment for MS using artificial nerve fibres. 01.2023
