Animal-free brain research
Today, there is a wide range of long-established as well as cutting-edge technologies based on human data or cells. These methods enable us to effectively understand the mechanisms of the human brain and develop specific therapies for treating neurological diseases as well as other conditions in humans.
Animal experiments in brain research
Despite its scientific and ethical shortcomings, animal experiments are still widely used in brain research in order to study neurological diseases, test therapies, and investigate the effects of drugs.
Mice are the most commonly used animals, but rats, zebrafish, nematodes, fruit flies, hamsters, guinea pigs, sheep, and primates are also involved in testing (1). The results of such experiments cannot, however, be transferred to the human organism, which calls the legitimacy of these methods into question (2).
In fact, up to 95% of drugs that are deemed successful in animal tests fail in subsequent clinical trials in humans (3). In the fields of neurology and psychiatry, this "failure rate" is even higher (4,5).
This is due to the substantial biological differences between humans and other animals, which make animal-based research ineffective, especially when studying complex systems like the human brain. In order to improve research efficiency and relevance, and to develop better medicine for humans, transitioning to animal-free methods is crucial.
Animal-free methods
Animal-free methods, such as population studies, electrophysiological methods, and imaging techniques, have been used in brain research for many years and have since provided human-relevant results for science and medicine. Additionally, innovative approaches like computer-based simulations and organoids allow for increasingly precise modeling of human neural processes, continuously opening up new, promising opportunities in research.
Population studies
Population studies have been used since the 19th century and allow for the collection of extensive datasets that reveal connections between genetic, environmental, and lifestyle factors, as well as various aspects of brain function. In these studies, groups of individuals are observed over years to decades, often based on common characteristics such as geographic location, ethnic background, or socioeconomic status. This long-term observation helps to understand the impact of lifestyle factors such as diet, exercise, or alcohol consumption on brain structure and function, as well as to identify risk factors for brain diseases.

Population studies provide information on the links between genetic, environmental and lifestyle factors and different aspects of brain function.
An important advantage of population studies is their ability to identify risk factors for diseases such as Alzheimer’s, Parkinson’s, and depression at early stages, prior to the onset of clinical symptoms. This enables early intervention, which can slow or even prevent disease progression.
An example of an important discovery from population studies is the finding that diet has a significant impact on cognitive function and the risk of cognitive impairments (6,7). For instance, a 2023 study revealed that individuals who consumed more highly processed foods experienced a greater decline in cognitive functions compared to those who consumed these foods less frequently (8).
Another key finding from a 2022 study highlights the link between social isolation, loneliness, and an increased risk of dementia, highlighting the crucial role of social connections for brain health (9).
Such population studies are essential for understanding epidemiological trends, identifying risk factors, and developing evidence-based prevention and treatment strategies.
Patient studies
Studies involving voluntary human participants have long been essential in brain research. As early as the 19th century, patient studies were conducted to explore the human brain. With the advent of non-invasive measurement techniques, these studies have since been systematically applied for research purposes.
Behavioral studies, neuropsychological tests, and imaging techniques allow researchers to collect valuable data on brain function and structure. Such investigations provide insights into the complexity of the human brain and the mechanisms underlying various neurological and psychiatric disorders.
A key advantage of these studies is that both healthy participants and patients with neurological or psychiatric conditions can provide direct feedback. Furthermore, these studies facilitate comparisons between healthy individuals and those with conditions, which helps to identify specific differences, enhancing our understanding of the disorders.
In certain cases, such as with patients suffering from severe epilepsy, electrodes are directly implanted in the brain to better manage their condition. These electrodes also allow researchers to measure the electrical activity of neurons in the brain directly, offering unique insights into brain functioning that would otherwise be impossible.
Through these measurements, scientists can gain deeper understanding on how the brain processes information and which areas are responsible for specific tasks. An example for that is a study in which researchers measured the activity of neurons in the medial temporal lobe – a region important for memory and learning – during simple arithmetic tasks. It was found that different brain areas exhibited distinct patterns when performing such tasks, shedding light on how the brain controls complex processes like arithmetic and how regions collaborate during these activities (10).
Special blood tests can also provide valuable information about pathological changes in the brain. For example, by analyzing extracellular vesicles in the blood, specific biomarkers can be identified that are associated with neurological and psychiatric disorders, such as schizophrenia, epilepsy, or depression (11).
Studies of brain tissue
Studies of brain tissue obtained from surgical procedures or from deceased individuals are of great importance (12). These tissue samples provide direct insights into the brain's anatomy and allow for the analysis of pathological changes such as tumors, inflammation, or neurodegenerative diseases like Alzheimer’s and Parkinson’s. Microscopic examinations and molecular analyses deepen our understanding of the biological foundations of these disorders and reveal potential therapeutic targets.
For example, in a post-mortem study, the role of the immune system in schizophrenia was analyzed. In patients with schizophrenia, particularly during psychotic episodes, an increased activation of microglia, the brain’s immune cells, was observed. These inflammatory processes could play a role in the development and progression of the disease, suggesting anti-inflammatory measures as potential therapeutic options (13).
Elektrophysiological methods
Electrophysiological methods allow for the measurement of brain activity without the need for invasive procedures. The so-called electroencephalography (EEG) records electrical signals from the brain and was first applied to humans over 100 years ago. In the 1960s, magnetoencephalography (MEG) was developed, which records the magnetic fields associated with the electrical brain signals. Both methods are used to investigate brain function - at rest as well as during task execution, for example when performing tasks on a monitor. These techniques work with both healthy individuals and those with brain disorders. Since EEG and MEG are completely harmless, they can be used repeatedly across all age groups without risk. This allows for long-term observations of the same individual, enabling us to track the development of brain activity over an extended period.
A modern technique, real-time source localization, enables the observation of brain responses in real time (14). This is especially useful in diseases like epilepsy, as it facilitates the detection of rapid and sudden changes in the brain during a seizure. Recent developments have also led to the creation of portable EEG devices (15). These devices allow for the measurement of brain activity in everyday settings, beyond the controlled environment of a laboratory. A study using these portable EEG devices has, for example, demonstrated that walking and navigating in natural environments affects our attention (16). Such findings are important for understanding how our brain functions in real-life situations.
In addition to EEG and MEG, other techniques are also used to precisely study the brain and its functions. One example is transcranial magnetic stimulation (TMS), which allows for the identification of critical brain areas prior to brain surgery and the 3D mapping of these areas onto imaging techniques. This technology plays a key role in pre-surgical diagnostics and neuronavigation in neurosurgery. Expanded diagnostic methods also include techniques such as video-oculography (VOG). This method uses special glasses with infrared cameras to record and analyze eye movements and disorders. It is primarily used to investigate dizziness and conditions of the cerebellum and brainstem. Along with caloric testing, in which the function of the horizontal semicircular canal in the inner ear is tested by warm and cold water irrigation, the labyrinth of the inner ear can be thoroughly examined.
The diverse use of various electrophysiological methods in research and clinical practice continuously expands our understanding of brain processes, leading to more precise diagnostics and the development of new therapeutic approaches.
Imaging techniques
Imaging techniques can be used for diagnosing and investigating neurological diseases.
Imaging techniques such as magnetic resonance imaging (MRI) and computer tomography (CT) are essential tools in modern medicine, widely used for the diagnosis and study of brain diseases. These techniques were established in clinical practice as early as the 1970s and 1980s.
MRI utilizes magnetic fields to generate detailed cross-sectional images of the brain, highlighting structural changes. In clinical settings, these images are typically produced with a resolution of 1 mm, whereas research applications can achieve resolutions down to 0.02 mm. A specialized form of MRI, functional MRI (fMRI), measures the blood flow to different brain regions to assess their activity during specific tasks. This enables the targeted examination of certain brain areas and the identification of abnormal brain activity.
CT, similar to MRI, creates cross-sectional images of the brain, but uses X-rays instead of magnetic fields. The resolution here is approximately 0.3 micrometers (0.0003 mm).
Both techniques can be easily combined with other diagnostic and research tools, such as positron emission tomography (PET). PET uses radioactively labeled substances to visualize specific brain functions, for example metabolism or medication effects.
In medicine, these techniques are frequently used to diagnose conditions such as inflammation, tumors, and diseases like dementia, multiple sclerosis, and strokes. In research, they help improve our understanding of brain disorders and develop more effective treatment methods. Thanks to technological advancements, increasingly finer details in the brain can now be detected, steadily expanding our knowledge of these diseases and laying the foundation for future therapeutic approaches (17).
For instance, a study found that functional MRI data combined with machine learning could predict the optimal parameters for deep brain stimulation in Parkinson’s patients (18). This could enhance the effectiveness of treatment and facilitate personalized therapy approaches.
Cell cultures
To study brain functions and processes at the cellular level, human brain cells can be cultured and investigated in the laboratory. These cells can be directly extracted from the brain, for example, during surgery, or obtained from other cell types, such as skin or blood cells. The latter process involves converting these cells into stem cells and then developing them into brain cells (19).
A key advantage of cell cultures is that they can be made from cells of patients, carrying information about their specific diseases. This enables the development of personalized cell cultures that can be used to model individual diseases and to specifically test drugs and therapies (20). This can, for example, help determine which medications are most effective for a particular person.
An important question in medical brain research is whether certain substances are harmful to the human brain. To address this, researchers have developed specialized cell cultures to test the effects of substances like pesticides or certain medications (21,22).
Overall, cell cultures in brain research help to investigate processes and interactions in a specific, individualized, and detailed manner. For example, in one study, cell cultures were used to examine the impact of a mutation associated with early-onset Parkinson’s disease on certain receptors (23). These findings may aid in a better understanding of the disease and the development of new treatment options. In another study, the molecular and genetic causes and effects of protein mislocalization associated with neurodegenerative diseases were analyzed in more detail, leading to the identification of a potential target for treating various forms of dementia (24).
Organoids
Organoids refer to millimeter-sized, three-dimensional structures from biopsies, body cells, or induced pluripotent stem cells that are cultivated in the laboratory. They consist of various organ-specific cell types and possess physiologically relevant properties of the actual organ. Brain organoids, for example, are composed of different types of nerve cells and mimic specific functions of the brain, such as memory and learning as well as sensory processing.
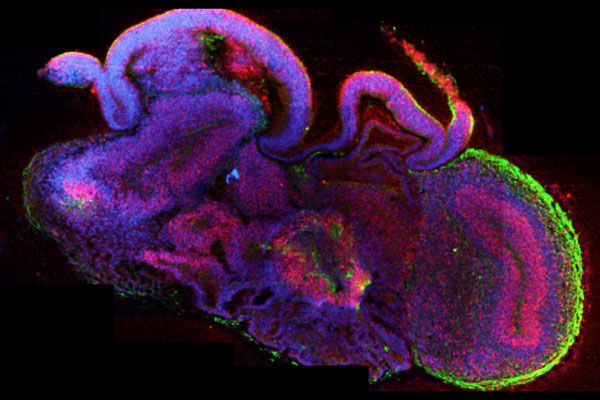
Mini brain grown in the laboratory.
These so-called "mini-brains" also allow for the observation of complex biological activities, such as brain development (25). They enable researchers to study how brain cells grow, connect, and communicate with each other. This is crucial for understanding how the brain works and identifying processes that may be disrupted in diseases such as Alzheimer’s or autism.
Furthermore, brain organoids can be used to test new medications in a complex, human-relevant, and patient-specific system. This allows researchers to determine which therapies are most suitable for patients without having to intervene directly in the human (26).
For example, in one study using brain organoids, it was discovered that the pesticide chlorpyrifos, in combination with a specific gene mutation, can increase the risk of developing autism (27). This demonstrates that the combination of certain chemicals and genetic changes can affect brain development and cause potential damage. In another study, researchers investigated how nerve cells in brain organoids from Parkinson's patients with a change in the GBA gene develop. It was found that these nerve cells cannot mature properly due to issues in the cell cycle, which may contribute to the development of Parkinson’s disease (28).
In addition to organoids, spheroids are also used in research. These are three-dimensional cell aggregates, but they usually consist of a smaller number of cell types and are capable of replicating basic tissue or organ functions, albeit in a less complex form. Spheroids are often used to study cell behavior, cellular interactions, and the effects of drugs in three-dimensional structures. In brain research, spheroids can be used to explore aspects of neural development and changes in disease models. Due to their reduced complexity compared to organoids, their production is faster, easier, and more economical. This makes them particularly useful for high-throughput screenings, where large quantities of samples can be quickly tested for active compounds or disease mechanisms (29).
By combining cell models with genetic engineering, specific genetic alterations can be introduced into the models to replicate relevant disease mechanisms and potential therapeutic targets more accurately. Furthermore, biobanks provide a valuable source of human cells that can be used as starting material for disease modeling. Utilizing these biobanks allows researchers to develop cell models based on a variety of genetic and clinical data, enabling the optimization of disease models (30).
Organ-on-a-chip technologies
Organ-on-a-chip technologies are tools specifically designed to observe physiological processes in vitro. Various organoids can be placed on such a chip and enclosed in an artificial fluid circuit that can, for example, simulate blood circulation (31).
So-called multi-organ chips allow for the combination of several organoids on a single chip, enabling the study of interactions and communication between these tissues (32).
This setup can also replicate signal transmission from the brain to other tissues, helping uncover the link between disruptions in this process and various neurological disorders.
One key application of organ-on-a-chip technologies in brain research is studying the blood-brain barrier (33). This barrier protects the brain from harmful substances while simultaneously allowing the transport of necessary nutrients. By simulating this barrier in organ chips, researchers can explore how drugs or toxins overcome the barrier. This is especially relevant for developing new therapies, as it provides insights into which compounds could be potentially harmful and how to transport substances safely to the brain.
Another important area of organ-on-a-chip applications is the exploration of changes in the brain under pathological conditions (34). Researchers can observe how nerve cells respond to various stress factors linked to conditions such as inflammation, neurodegenerative diseases, or cancer. By investigating the interactions between cells and the impact of external factors, crucial mechanisms contributing to disease development can be identified.
Overall, organ chips allow for the study of complex biological processes in a controlled environment, aiding in the discovery of new insights into the functioning of the human brain.
For instance, in one study, a multi-organ chip was developed to connect the microphysiological systems of the gut, liver, and brain to better understand how these organs interact in neurodegenerative diseases. The study found that changes in the gut and liver can influence neurodegenerative processes in the brain, highlighting the significance of the gut-brain-liver axis in the research and treatment of such diseases (35).
3D bioprinting
Through the use of 3D bioprinting, complex brain structures can be realistically replicated. This innovative technology utilizes living cells to create tissue that simulates both the architecture and function of the human brain. In addition, 3D bioprinting offers the flexibility to precisely control the arrangement of various cell types within the tissue (36). This allows for the accurate replication of different pathological conditions, such as injuries and damage, enabling the observation of their effects on cell function. This is particularly valuable for understanding neurodegenerative and complex diseases.
For example, through 3D bioprinting, a perfused neuroblastoma tumor model was developed on a chip. This platform can be used to study the formation of blood vessels in tumors, tumor spread, as well as for drug testing (37).
Computer programs/AI
Artificial intelligence (AI) and computer programs are playing an increasingly important role in brain research. These technologies facilitate the analysis of complex data, the identification of patterns, and the discovery of new insights into the human brain. By processing information from population and patient studies, mathematical models optimize disease diagnostics and provide valuable insights into specific conditions and potential therapies.
A significant application of AI in brain research is the analysis of imaging techniques such as MRI and CT scans (38). AI algorithms can quickly and accurately assess images to identify changes in brain structure or function, enabling the early detection of diseases like Alzheimer's, strokes, or tumors.
Additionally, AI is used to analyze large datasets from clinical studies. With the help of machine learning, connections between genetic factors, lifestyle, and the onset of brain diseases can be uncovered, which can contribute to the development of personalized treatment approaches (39).
Another key aspect is the simulation of brain activities. Computer programs can model neural networks, investigating the behavior of nerve cells and their interactions. These models help deepen the understanding of learning and memory processes and evaluate the effects of therapies. By combining AI with brain organoids, even molecular and cellular aspects of learning, memory, and cognitive abilities can be modeled and studied. This forms the core approach of an innovative research field known as "organoid intelligence" (40).
In toxicology, computer programs and AI can also be used to study the effects of chemicals on the human brain and other organs (41,42). AI-driven models can predict how certain substances may damage nerve cells or impair brain function, allowing potentially harmful substances to be identified more quickly and their safety profiles assessed more accurately.
In one study, for example, AI was applied to detect specific protein patterns in the blood of individuals with autism spectrum disorders. Through this analysis, specific biomarkers associated with autism were identified, improving the diagnosis and understanding of the disorder (43). In another investigation, AI models were used to analyze large volumes of imaging and patient data, enabling the early detection of Alzheimer's disease (44). Early detection of the disease is crucial, as it allows for more timely intervention.
Conclusion
The transition to animal-free methods in brain research is an important and necessary step to advance scientific progress. By using human-based technologies and models, not only is the relevance of the research to humans increased, but it also creates the opportunity to develop more precise and effective treatment methods for neurological diseases, without causing harm to either humans or animals. These approaches reflect a commitment to both ethical considerations and scientific innovation, providing a platform for developing safer, more efficient therapies tailored to human physiology.
16.12.2024
Leyla Fox, Neuroscientist
References
- Homberg J.R. et al. The continued need for animals to advance brain research. Neuron 2021; 109(15):2374–2379
- Neumann, G. Scientific arguments against animal experiments, 02.08.2023 (accessed 05.11.2024)
- Sun D. et al. Why 90% of clinical drug development fails and how to improve it? Acta Pharmaceutica Sinica B 2022; 12(7):3049–3062
- Biomedtracker. Clinical development success rates 2011-2020, Biomedtracker, 2020
- Biomedtracker. Clinical Development Success Rates 2014-2023, Biomedtracker, 2024
- Spencer S.J. et al. Food for thought: how nutrition impacts cognition and emotion. npj Science of Food 2017; 1(1):7
- Puri S. et al. Nutrition and cognitive health: A life course approach. Frontiers in Public Health 2023; 11
- Gomes Gonçalves N. et al. Association Between Consumption of Ultraprocessed Foods and Cognitive Decline. JAMA Neurology 2023; 80(2):142–150
- Shen C. et al. Associations of Social Isolation and Loneliness With Later Dementia. Neurology 2022; 99(2):e164–e175
- Kutter E.F. et al. Neuronal codes for arithmetic rule processing in the human brain. Current Biology 2022; 32(6):1275-1284.e4
- Smirnova L. et al. Blood extracellular vesicles carrying brain-specific mRNAs are potential biomarkers for detecting gene expression changes in the female brain. Molecular Psychiatry 2024; 29(4):962–973
- Grubisha M.J. et al. Investigating Post-translational Modifications in Neuropsychiatric Disease: The Next Frontier in Human Post-mortem Brain Research. Frontiers in Molecular Neuroscience 2021; 14
- De Picker L.J. et al. Immune environment of the brain in schizophrenia and during the psychotic episode: A human post-mortem study. Brain, Behavior, and Immunity 2021; 97:319–327
- Muñoz-Gutiérrez P.A. et al. Localization of Active Brain Sources From EEG Signals Using Empirical Mode Decomposition: A Comparative Study. Frontiers in Integrative Neuroscience 2018; 12
- Casson A.J. Wearable EEG and beyond. Biomedical Engineering Letters 2019; 9(1):53–71
- Liebherr M. et al. EEG and behavioral correlates of attentional processing while walking and navigating naturalistic environments. Scientific Reports 2021; 11(1):22325
- Yen C. et al. Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023; 13(7):1472
- Boutet A. et al. Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning. Nature Communications 2021; 12(1):3043
- Tao Y. et al. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell 2016; 19(5):573–586
- Maldonado-Soto A.R. et al. Stem Cells in the Nervous System. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists 2014; 93(11 Suppl. 3):132–S144
- Klima S. et al. Examination of microcystin neurotoxicity using central and peripheral human neurons. ALTEX - Alternatives to animal experimentation 2021; 38(1):73–81
- Loser D. et al. Human neuronal signaling and communication assays to assess functional neurotoxicity. Archives of Toxicology 2021; 95(1):229–252
- Sarasola L.I. et al. The ADORA1 mutation linked to early-onset Parkinson’s disease alters adenosine A1-A2A receptor heteromer formation and function. Biomedicine & Pharmacotherapy 2022; 156:113896
- Brown A.-L. et al. TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature 2022; 603(7899):131–137
- Shou Y. et al. The Application of Brain Organoids: From Neuronal Development to Neurological Diseases. Frontiers in Cell and Developmental Biology 2020; 8
- Zhou J.-Q. et al. Brain organoids are new tool for drug screening of neurological diseases. Neural Regeneration Research 2023; 18(9):1884
- Modafferi S. et al. Gene–Environment Interactions in Developmental Neurotoxicity: a Case Study of Synergy between Chlorpyrifos and CHD8 Knockout in Human BrainSpheres. Environmental Health Perspectives 2021; 129(7):077001
- Rosety I. et al. Impaired neuron differentiation in GBA-associated Parkinson’s disease is linked to cell cycle defects in organoids. npj Parkinson’s Disease 2023; 9(1):1–16
- Fang Y. et al. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discovery 2017; 22(5):456–472
- Botti G. et al. Organoid biobanks as a new tool for pre-clinical validation of candidate drug efficacy and safety. International Journal of Physiology, Pathophysiology and Pharmacology 2021; 13(1):17–21
- Lee S.H. et al. Advances in dynamic microphysiological organ-on-a-chip: Design principle and its biomedical application. Journal of Industrial and Engineering Chemistry 2019; 71:65–77
- Park S.E. et al. Organoids-on-a-chip. Science 2019; 364(6444):960–965
- Li M. et al. Blood–brain barrier microfluidic chips and their applications. Organs-on-a-Chip 2023; 5:100027
- Zeng Y.-L. et al. On-chip modeling of physiological and pathological blood-brain barrier microenvironment for studying glial responses to neuroinflammation. Nano Today 2023; 52:101947
- Trapecar M. et al. Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases. Science Advances 2021; 7(5):eabd1707
- de la Vega L. et al. 3D bioprinting models of neural tissues: The current state of the field and future directions. Brain Research Bulletin 2019; 150:240–249
- Scarian E. et al. Patients’ Stem Cells Differentiation in a 3D Environment as a Promising Experimental Tool for the Study of Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences 2022; 23(10):5344
- Monsour R. et al. Neuroimaging in the Era of Artificial Intelligence: Current Applications. Federal Practitioner 2022; 39(1):14-20
- Rudroff T. Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges. Neurology International 2024; 16(4):805–820
- Smirnova L. et al. Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Frontiers in Science 2023; 1
- Alam El Din D.-M. et al. Organoid intelligence for developmental neurotoxicity testing. Frontiers in Cellular Neuroscience 2024; 18
- Furxhi I. et al. Predicting In Vitro Neurotoxicity Induced by Nanoparticles Using Machine Learning. International Journal of Molecular Sciences 2020; 21(15):5280
- Hewitson L. et al. Blood biomarker discovery for autism spectrum disorder: A proteomic analysis. PLOS ONE 2021; 16(2):e0246581
- Kavitha C. et al. Early-Stage Alzheimer’s Disease Prediction Using Machine Learning Models. Frontiers in Public Health 2022; 10
