Animal experiments for vaccineproduction and testing
- an overview
Vaccines are immunological medicinal products that contain parts (antigens) of bacterial or viral pathogens, or induce their production in the body, thus triggering an immune response that leads to protective immunity against the relevant pathogen. Today, more than 80% of the world's population is prophylactically vaccinated against some serious or fatal diseases such as diphtheria, tetanus, whooping cough, and poliomyelitis (1). However, the production of most vaccines involves an enormous amount of animal use and animal testing for a variety of reasons, most of which are regulatory.
The following text describes the key aspects of animal experimentation and animal use for the manufacturing and testing of vaccines for human use. We take a detailed look at 20 of the most commonly used human vaccines and explain whether and which animal tests are carried out for their regular batch testing, and whether animal components are used in the manufacturing process. Similar methods are also used for vaccines for animals - but they are not the subject of this study. Here, we also do not discuss the efficacy and vaccine damage.
Doctors Against Animal Experiments does not give recommendations whether or not to get vaccinated against any disease or with any vaccine. The aim of this text is to provide information on the situation regarding vaccine manufacturing and testing, and the associated animal use. Our organization is not against vaccines and medicines, only against the way they are usually tested, namely on animals. We are convinced that without animal experiments, vaccines and drug development would not only be possible, but also much better, faster and more efficient, since human-based test methods, in contrast to animal experiments, provide information relevant to humans.
The different types of vaccines
The first main distinction is between live and inactivated vaccines. In recent years, an increasing number of gene-based vaccines have also been developed and marketed. These can be described as a single group (2).
Live vaccines consist of chemically or physically attenuated pathogens (viruses or bacteria) that replicate in the human body for some time but cannot cause disease in healthy individuals. Examples of live vaccines include the measles-mumps-rubella combination vaccine, and the shingles and varicella vaccines.
Inactivated vaccines are based on dead viruses or bacteria. These can be whole pathogens (so-called whole pathogen inactivated vaccines such as hepatitis A and TBE vaccines), as well as individual parts of the pathogens that serve as antigens (so-called subunit, split, and component vaccines such as certain influenza and pneumococcal vaccines). Furthermore, some vaccines consist of inactivated bacterial toxins (so-called "toxoids", e.g. the diphtheria and tetanus vaccines).
With gene-based vaccines (mRNA, DNA, and vector vaccines), the genetic information of the pathogen for the construction of the desired antigens is introduced into the body and the antigens are produced by the vaccinated person's own cells. The genetic information can be in the form of DNA (currently no approved vaccines for humans), mRNA (COVID-19 vaccines from BioNTech/Pfizer and Moderna) or harmless carrier viruses with the DNA for the antigen (so-called vector vaccines, e.g. the Ebola vaccines of the company Janssen and the COVID-19 vaccines from AstraZeneca and Janssen).
How are vaccines regulated?
The development, manufacture, and testing of vaccines is subject to strict controls, both by the manufacturer and by control institutions. Worldwide, the World Health Organization (WHO) requirements govern general aspects of entire vaccine groups, as well as specific products and vaccine components (3). In the EU, the manufacture and testing of each type of vaccine for each disease (e.g. live and inactivated influenza vaccines) must comply with the relevant European Pharmacopoeia regulations. Additional requirements and guidelines may also apply at the national level; in Germany, these are the requirements of the German Pharmacopoeia, the Medicinal Products Act and the Medicines Testing Guidelines. In Germany, the Paul Ehrlich Institute (PEI) is responsible for vaccines and monitors their quality, efficacy, and safety (4).
Both the international WHO requirements, and the European and German regulations require several animal tests, among others, for the safety and efficacy testing of the finished vaccines and some of their components.
Animal testing in vaccine development
Worldwide, an estimated 10 million animals are used annually for the development and testing of vaccines and other immunological drugs.
During vaccine development, a wide range of animal studies are typically used for a variety of purposes before the vaccines are allowed to be tested in what are known as human clinical trials. Clinical trials consist of three consecutive test phases with healthy volunteers and patients, in which drug or vaccine candidates are tested to determine their safety and efficacy. Whether a drug or a vaccine is approved for the market is decided based on these results. In phase 4, the efficacy and possible side effects are monitored after the compounds have been marketed. The animal tests that are done before clinical trials ("preclinical phase") are often done in different animal species to determine, for example, whether the vaccine candidate leads to a desired immune response in the animals, whether it protects them from infection with the corresponding pathogen, whether the animals develop disease symptoms and can infect other animals, whether and what side effects occur after single and multiple vaccine administration, how the vaccine is distributed in the different organs, whether the vaccine is harmful to the environment, etc.
Exactly which animal tests are done is decided by the vaccine manufacturers based on international and European regulations, and after consultation with the relevant regulatory authorities. In doing so, different manufacturers may conduct multiple and different animal studies for testing similar aspects and similar vaccines. The novel COVID-19 vaccines from BioNTech/Pfizer and Moderna are based on the same principle (mRNA vaccines) and act against the same pathogen (SARS-CoV-2); both vaccines were tested on mice, rats, and monkeys in adherence with WHO specifications, but Moderna's vaccine was additionally tested on hamsters (5,6). Similarly, the two Sars-CoV-2 vector vaccines from AstraZeneca and Janssen were tested on mice and monkeys; AstraZeneca's vaccine was additionally tested on pigs and ferrets, and Janssen's was tested on hamsters and rabbits (7,8).
Animal use in vaccine production

450-500 million embryonated eggs are used annually worldwide for the production of vaccines. Image source: flick.com/Governo do Estado de Sao Paulo (cc-by-2.0)
Not only the development but also the production of most vaccines involves an enormous use of animals. Table 1 summarizes the production systems approved in the EU for 20 commonly used vaccines for human use. Many vaccines are produced in incubated chicken eggs because some viruses, such as the influenza virus, used to produce vaccines, can replicate in the egg or chicken embryo. These eggs contain half-developed embryos that can feel pain and are killed during vaccine preparation. According to a WHO estimate, 450-500 million eggs are used annually worldwide for vaccine manufacturing, a large proportion of which goes into the production of influenza vaccines (9). In addition to the ethical problems, there are also health concerns with these production methods, because the viruses used as live vaccines mutate rapidly within the eggs, which can greatly reduce vaccine efficacy (9,10). Such viral mutations of the influenza virus are likely the reason why the 2018 influenza vaccine had an efficacy of only 36% (9).
Other vaccines are propagated in animal cells: these are either cells taken directly from the animal ("primary cells") or "immortalised cell lines" that are propagated and used over several years and decades after being obtained once from an animal. There are vaccines, such as the rubella vaccine, that are produced in human cells. Furthermore, vaccines against bacterial pathogens, such as the diphtheria, tetanus, or meningococcal vaccines, are grown in bacterial cultures. Some vaccines, such as those against hepatitis B or the human papilloma virus, are produced in yeast. The mRNA vaccines against COVID-19 are generated entirely in vitro, i.e., in test tubes, with bacterial cultures used only for some intermediate steps.
Tobacco plants represent a relatively new but promising and animal-free method for vaccine production. Vaccines can be grown and prepared in large quantities in the fast-growing plants. Because tobacco plants constitute a completely different environment for human viruses, there are no undesirable reactions between the plants and the viruses, and accordingly no changes in vaccine structure. The tobacco manufacturer British American Tobacco is developing an influenza vaccine and a COVID-19 vaccine using this technology. Both vaccines are already being tested in human clinical trials and may be stable at room temperature, which would offer significant advantages (11).
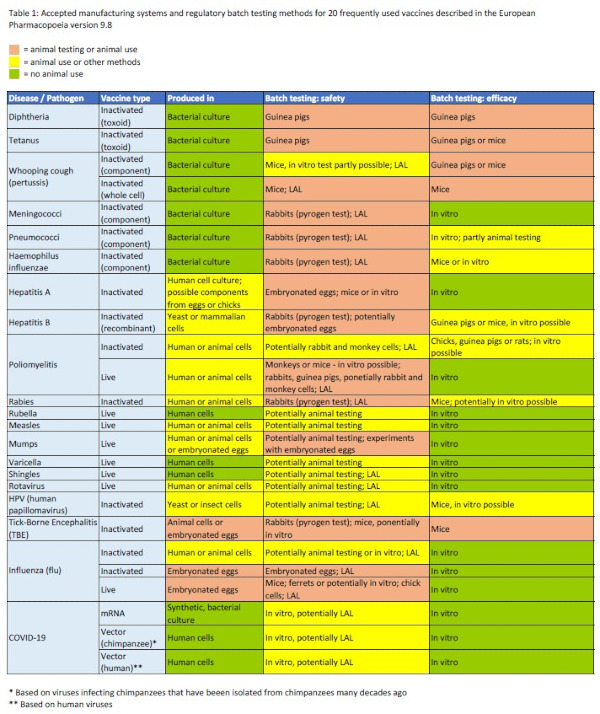
Table 1: Approved manufacturing systems and regulatory batch testing methods for 20 commonly used vaccines described in the European Pharmacopoeia version 9.8.
Are there vegan vaccines?
In the course of routine vaccine production, animal materials such as the infamous fetal calf serum (FCS), cow's milk, gelatine, enzymes, meat extracts, eggs, and feathers are often used. Many of these are used as excipients or carriers, and some are used in the devices and packaging materials. Often, these components are removed before the final steps of vaccine production so that the finished vaccine contains no or few ingredients of animal origin because they can pose a potential health risk to humans. However, those interested in a vegan vaccine or vaccine with minimal animal use for ethical reasons will certainly want to know if animal materials were used in the manufacturing process, even if they are not present in the finished vaccine.
Fetal calf serum (FCS): scientifically and ethically unjustifiable
Fetal calf serum (FCS), extracted from the blood of unborn calves, is often used as the "gold standard" in the composition of culture media for growing a number of cells. FCS presents both ethical and scientific problems because its production involves great animal suffering, and because it can cause reactions in human-based models that do not naturally exist in the human body. Several culture media exist now that contain human platelet lysate (hPL) instead of FCS, which is produced from expired blood donations and has many ethical and scientific advantages.
More about FCS >> (Link fehlt)
To provide an overview of which of the 20 most commonly used vaccines are produced using animal materials, we evaluated official information from the European Medicines Agency (EMA) (Table 2). Since 1995, EMA issues the European Public Assessment Reports (EPARs) on all drugs that are approved in the EU (12). The EPAR reports describe, among other things, the production systems and whether and which animal components are used for manufacturing. In Table 2, red indicates when animal materials such as eggs or primary animal cells are used. Marked with yellow are vaccines for which it is not clear whether and which animal materials are used in their production or those that are propagated in immortalised animal cell lines that originally come from animals but do not cause permanent animal use. Vaccines that are produced in an entirely animal-free manner are marked with green. There are several vaccines for which EPAR reports are not available, presumably because they were licensed before 1995. For example, this is the case of all rabies and TBE (tick-borne encephalitis) vaccines.
Unfortunately, a full range of animal testing is routinely performed for most vaccines, including those produced without animal materials.
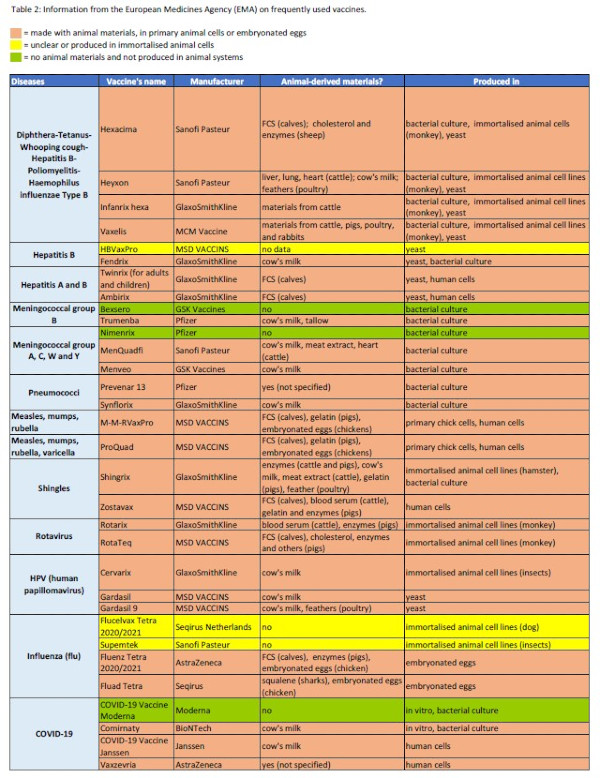
Table 2: Information from the European Medicines Agency (EMA) on the use of animal cells, embryonated eggs,or other animal materials for commonly used vaccines.
Animal experiments for vaccine batch testing
Due to their biological nature, the quality and efficacy of vaccines can vary during production. Therefore, unlike most other medicines, vaccines are not only subject to a one-time control during the drug development process, but each production unit (batch) is tested in the so-called batch tests before it is allowed to be released on the market (13). Thus, the safety and efficacy of each vaccine batch is tested, which often involves numerous excruciating animal tests. According to one estimate, 10 million animals are used annually worldwide in the development and testing of vaccines and other immunologic drugs, approximately 80% of which are used in routine batch testing (14). One reason for the high animal numbers is that, as a rule, not only the finished vaccines but also the active substance and some excipients (e.g. adjuvants) are routinely tested. The biological components of the production system, such as cells or embryonated eggs, are also subject to regular batch testing. Moreover, multiple dilutions of both the finished vaccine and its components are often tested in animals, further increasing animal numbers. Furthermore, in addition to the animal experiments performed by vaccine manufacturers as part of batch testing, some animal trials are repeated at the Official Medicines Control Laboratories (OMCLs), i.e., twice as many animals are used in these cases (15).
Table 1 (see above) summarizes the animal tests required by the European Pharmacopoeia for each of the 20 most common vaccines (16).
Efficacy batch testing of most live vaccines, such as the measles-mumps-rubella-varicella vaccine and the shingles vaccine, is not based on animal experiments but involves various in vitro testing methods. The efficacy of some inactivated vaccines, such as the meningococcal and influenza vaccines, and gene-based vaccines (e.g. the COVID-19 vaccines) is also analysed without the use of animals. In contrast, extensive animal trials are performed for routine efficacy testing of most inactivated vaccines. For example, more than 100 animals suffer and die for demonstrating the efficacy of each batch of diphtheria, tetanus, pertussis, and rabies vaccines (14). Mice and guinea pigs are most commonly used, and sometimes also chicks and rats, such as for the poliomyelitis inactivated vaccine.
So-called challenge studies are typical: several groups of animals are injected with different dilutions of the vaccine batch as well as with a reference vaccine. Some animals serve as "controls" and are not vaccinated. After a period of time, the animals are exposed to a very high dose of the pathogen (challenge), which causes severe disease symptoms such as convulsions, paralysis, and respiratory distress in the control animals and in the animals vaccinated with low dilutions, leading to the animals' agonizing death. The goal of these experiments is to determine the LD50 (Lethal Dose 50 = the vaccine dilution at which 50% of the animals die). Alternatively, for some vaccines, the animals cannot be exposed to the pathogen, but the level of antibodies is determined in blood samples from the vaccinated animals. This method is not quite as distressing as the classic LD50 determination, and is preferred for vaccines for which it is approved. However, the animals are killed at the end of those tests as well. In the efficacy testing of some vaccines, the European Pharmacopoeia allows the replacement of the described animal trials in whole or in part by "a validated in vitro method." Unfortunately, it is unclear whether and how often such methods are actually used by vaccine manufacturers. In 2019, nearly 140,000 animals were used for the efficacy batch testing of vaccines and other immunological drugs in Germany, including mice, rats, guinea pigs, hamsters, rabbits, and others (17).
Safety batch testing is also required by law and is intended to ensure the safety of each vaccine production unit. Here, various and numerous animal trials are also very often mandated by the European Pharmacopoeia (Table 1). Similar to efficacy testing, these animal experiments are also performed at several stages of vaccine production, e.g. to test the safety of the active ingredient and the finished vaccine.
Depending on the vaccine type and technology, different safety parameters are tested in specific animal studies. For toxoid vaccines, such as those for diphtheria and tetanus, whose active substance consists of an inactivated bacterial toxin ("toxoid"), the absence of the active toxin is determined by injecting the toxoid into animals (e.g. guinea pigs) at a higher dose than in the finished human vaccine. For several weeks, the animals are observed to see if they develop symptoms of poisoning or die.

In the pyrogen test, rabbits are crammed into narrow boxes.
Almost all vaccines must also be tested for the absence of pyrogens or endotoxins. Pyrogens are fever-inducing substances that can occur due to contamination with bacteria, viruses, fungi or artificial materials such as plastic or metal particles during vaccine production. Endotoxins are bacterial components. In the "classical" pyrogen test on rabbits, the animals are placed in narrow boxes so that they cannot move, the test substance (vaccine or vaccine component) is injected into an ear vein and their body temperature is recorded by a thermometer in the anus. This test is associated with enormous stress for the rabbits, and they sometimes experience fever, breathing difficulties and shock. In Germany alone, 6,457 rabbits were used for pyrogen testing of vaccines and other medicines in 2019 (17).
An officially approved "alternative" of the pyrogen test in rabbits is the limulus amoebocyte lysate test (LAL test), which checks for the presence or absence of endotoxins. Blood from wild-caught horseshoe crabs is employed in this test. The animals are caught on a large scale, and taken to specialised laboratories where a third of their blue blood is drawn without anaesthesia. Many animals do not survive the procedure. As this test involves great animal suffering, it is not an ethically justifiable "alternative" to the pyrogen test on rabbits. In the rFC test (recombinant factor C), the molecule on which the LAL test is based is obtained synthetically instead of from horseshoe crabs. Since January 2021, the rFC test has been approved as an LAL test alternative in the European Pharmacopoeia (18).
The monocyte activation test (MAT test) is an animal-free test method analysing the presence of pyrogens based on human blood cells (monocytes) that has been approved in the European Pharmacopoeia since 2010. The MAT test not only has ethical advantages, but it can be used to detect a much wider range of fever-inducing contaminants than the rabbit or LAL test. DAAE calls for the use of the non-animal rFC and MAT tests instead of the rabbit pyrogen test and the LAL test.
Detailed information on the pyrogen test on rabbits, LAL-, MAT- and rFC-Test >>
Other safety batch tests are performed to determine the sterility of the biological component of the manufacturing system, e.g. animal cells or embryonated eggs. In 2019 in Germany, 25,013 animals were used for safety batch testing of vaccines and other immunological medicinal products in addition to the 6,457 rabbits used for pyrogen testing (17).
Successes in removing animal experiments from vaccine batch testing
Many organisations and programmes in Europe and worldwide have been working for decades to reduce the use of animals in vaccine batch testing. There are four main drivers for these efforts: 1. animal welfare, because very large numbers of animals are used in these cruel tests; 2. scientific reasons, because many of the common animal experiments give highly variable, unreliable, and non-transferable results; 3. economic reasons, because animal tests are usually more expensive, laborious, and lengthy than in vitro methods; and 4. regulatory reasons, because EU law requires that animal experiments be reduced and replaced where possible (19). As a result, some animal tests have been removed or replaced by in vitro methods, and some non-animal methods have been listed in the European Pharmacopoeia in addition to the usual animal experiments (20). Some positive examples in recent years are:
- In 2014, an immunological in vitro method (ELISA) was officially recognised to determine the antigen concentration of the hepatitis A vaccine, which was previously tested on mice. Unfortunately, the mouse test has not been removed from the European Pharmacopoeia, which means that each manufacturer is still free to choose whether to use the animal or the non-animal test method.
- In 2015, some animal testing on guinea pigs and adult mice for testing for certain vaccine impurities was removed from the European Pharmacopoeia, and the previously common use of baby mice and embryonated eggs for these purposes was restricted (20).
- In 2016, the chapter in the European Pharmacopoeia on the MAT test was revised to promote the use of this non-animal method instead of the rabbit pyrogen test (20).
- In 2017, the test for abnormal toxicity in mice and guinea pigs was removed from the European Pharmacopoeia for all new vaccines after its validity had been questioned for more than two decades (13,19,20). This test has been around for over 100 years, and used to be a routine test for all vaccines.
- In 2018, a cell culture-based in vitro method was approved by the European Pharmacopoeia to replace some mouse experiments for safety testing of the active ingredient and, in some cases, the finished pertussis vaccine (19,20).
- In 2019, three safety trials on guinea pigs for the tetanus vaccine were removed from the European Pharmacopoeia (20). However, further similar tests on guinea pigs are unfortunately still mandatory during the manufacturing process of this vaccine and are still being conducted.
- Since 2021, the rFC test has been recognised as an alternative to the LAL test in the European Pharmacopoeia (18).
- The European Pharmacopoeia allows the use of in vitro methods instead of the usual animal experiments for efficacy testing of the inactivated poliomyelitis, rabies, hepatitis A, hepatitis B, Haemophilus influenza and papillomavirus vaccines (19).
- Over the last four decades, further improvements in regulations have been made, such as the replacement of the neurovirulence test in monkeys for live poliomyelitis vaccines with an in vitro method (13).
Innovative vaccine testing without animals

Organ-on-a-chip technology can be used to grow human mini lymph nodes for vaccine research. Image source: LuchscherF/stock.adobe.com
While the legal introduction of non-animal methods instead of the usual animal trials for vaccine batch testing is a lengthy process, the scientific field of these innovative, human-relevant technologies is growing rapidly.
- Human mini lymph nodes, so-called lymph node organoids grown from human cells, such as those of the American company Prellis Biologics or the German company ProBioGen AG, are used to analyse the human immune response and antibody production to various substances and vaccines (21,22).
- Lymph-node-on-a-chip systems, which can be treated with an influenza vaccine, for example, can be used to study patient-specific immune responses, and to analyse the efficacy and safety of vaccines (23).
- Based on human blood and immune cells, the modular MIMIC system can serve as a preliminary screen for efficacy testing of potential vaccine formulations, and can be used to measure immune system stimulation by vaccines (24).
- A combination of specific cell types and certain biochemical methods is able to determine the efficacy of the TBE vaccine and could thus replace the corresponding animal tests for batch testing of this vaccine (25).
- Modern, powerful computer programmes are also being used for vaccine analysis. For example, a computer algorithm was recently developed to predict the efficacy of COVID-19 vaccines (26).
These and other examples clearly show that there is no shortage of modern, human-relevant, non-animal methods that can be used to develop and test vaccines and ensure their efficacy and safety.
Why are animal experiments still being done for vaccine batch testing?
The legally required validation and acceptance of non-animal test methods, and the replacement of "classical" animal tests which have been carried out for decades is a complex, lengthy process. Many standard animal experiments in batch testing of vaccines were introduced several decades or even a century ago. Although these outdated animal procedures have never been validated, and are much less reliable than today's modern non-animal test methods, they have been considered the "gold standard" for decades and are still carried out without being questioned. With the high quality standards and good manufacturing practices (GMP) that ensure uniformity in vaccine production today, animal testing seems like something from another era. Animal experiments are often carried out even when non-animal methods for the same purpose have long been available and regulatory accepted. A tragic example is the MAT test, which has been approved by the European Pharmacopoeia as an alternative to the pyrogen test on rabbits since 2010. Despite this, more than 6000 rabbits are used annually in Germany for pyrogen testing (17). The practical implementation of the improved regulatory guidelines is confronted with numerous hurdles.
One major problem is the absence of a worldwide harmonisation of requirements for vaccine testing. This means that some animal tests are required in some countries and not in others, or that (sometimes minimally) different animal tests are required in different countries. Vaccine manufacturers who want to sell their products worldwide are thus forced to conduct these additional animal tests. For example, abnormal toxicity testing on mice and guinea pigs, which has not been required in the US or EU since 2015 and 2017 respectively, is still required by law in many other countries with large, lucrative markets such as China, Japan, Mexico, and Russia (27). International programmes such as the Animal-Free Safety Assessment (AFSA), a collaboration between the Humane Society International (HSI) and several companies, aim to align (harmonise) regulatory testing guidelines for vaccine batch testing globally (28).
Another issue is the complexity of the regulatory validation and approval processes. Validation comprises three phases that can take 10-15 years or even longer, and involves a great deal of work such as multiple testing of the method both internally and at 10-20 international laboratories, submission of extensive documentation, and obtaining licences (29). This burdensome procedure slows down the development of non-animal test methods, and discourages vaccine manufacturers from using them. This is one of the main reasons why pharmaceutical companies do not fully implement the animal-free methods recognised in the European Pharmacopoeia (19). For the diphtheria vaccine, for example, an animal-free method is approved instead of a toxicity test on guinea pigs in the European Pharmacopoeia. However, due to country-specific requirements, the replacement of this animal test has only been partially implemented (19).
Furthermore, it is problematic that many animal experiments cannot be replaced one-to-one by an animal-free method, which is often required by the relevant authorities. Instead, it would make sense to determine suitable measurement parameters for the non-animal method. For some purposes, a combination of several methods may also be useful. However, this is not (yet) accepted by most authorities.
For older vaccines, some manufacturers have been carrying out the mandatory batch tests for years with the help of certain animal experiments, and have established production chains and testing processes. In these cases, it may be economically unfavourable, at least initially, to change the existing testing systems to replace some animal tests. The same is true for switching from a production system based on animal materials such as embryonated eggs to an animal-free manufacturing process.
Another reason for the slow progress is that non-animal methods often need to be validated on a product-specific basis, i.e. for each individual vaccine or test substance. This is also the case with the MAT test, which has to go through a validation process every time a vaccine manufacturer wants to use it for a new product (30). In contrast, the rabbit pyrogen test may be used for all substances without the need for such additional validation. This double standard means that it is more convenient for manufacturers to continue with the existing animal tests instead of going through the lengthy validation process for each product.
Conclusion / Our demands
Vaccine research is a field that has been developing for centuries, and whose beginnings are based on observations and experiments with humans. Vaccine development and production are subject to strict international and governmental guidelines, often involving enormous animal use and many animal experiments. Every year, millions of animals are subjected to excruciating experiments as part of the regulatory batch testing for safety and efficacy of each vaccine production unit. These are mostly outdated animal tests that have been considered the "gold standard" for decades, even though there are many more effective non-animal testing methods available today. In recent decades, some successes have been achieved by replacing certain animal experiments for vaccine batch testing with non-animal methods, or eliminating them without replacement. Nevertheless, regulatory acceptance of non-animal test methods is an incredibly lengthy process with many obstacles. One major problem is the different batch testing guidelines in different countries, which result in many animal tests being done twice or more.
Doctors Against Animal Experiments advocates for animal-free vaccine production, the suspension of animal experiments, and the consistent implementation of regulatory approved non-animal methods for vaccine batch testing. Animal experiments for trials where non-animal test methods are already regulatory approved must be removed from the European Pharmacopoeia immediately. We demand from the German and European authorities to enable and facilitate the use of human-relevant, non-animal test methods instead of outdated animal tests. Furthermore, the development and validation of these methods must be much better financed, and the corresponding validation processes accelerated.
01 June 2021
Dr. Dilyana Filipova
References
- WHO: Immunization coverage, 15.7.2020
- Deutsches Grünes Kreuz für Gesundheit e.V.: Hintergrundwissen: Die verschiedenen Impfstoff-Typen, 13.10.2020
- WHO: Vaccine regulation
- Paul-Ehrlich-Institut: Impfstoffe, 21.11.2019
- European Medicines Agency: Comirnaty, 21.12.2020
- European Medicines Agency: COVID-19 Vaccine Moderna, 4.1.2021
- European Medicines Agency: COVID-19 Vaccine AstraZeneca, 25.1.2021
- European Medicines Agency: COVID-19 Vaccine Janssen, 5.3.2021
- Precision Vaccinations: 500 million easter eggs could be saved by the FDA, 29.3.2018
- Zost SJ et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. PNAS 2017; 114(47):12578–12583
- British American Tobacco: BAT progresses COVID-19 candidate vaccine into Phase I human clinical trials, 16.12.2020
- Papathanasiou P et al. Transparency in drug regulation: public assessment reports in Europe and Australia. Drug Discov Today 2016; 21(11):1806–1813
- Cußler K et al. Alternativen zu Tierexperimenten: wissenschaftliche Herausforderungen und Perspektiven, Spektrum Akademischer Verlag, 1996, S. 163–188
- Halder M Three Rs potential in the development and quality control of immunobiologicals. ALTEX 2001; 18 Suppl 1:13–47
- EDQM: Batch release for human biologicals: vaccines, blood and plasma derivatives
- Europäisches Arzneibuch 9.8, Deutscher Apotheker Verlag, 2019
- BMEL: Verwendung von Versuchstieren im Jahr 2019, 12.8.2020
- Deutschmann S et al. General chapter on the rFC test adopted by the European Pharmacopoeia Commission. Eur Pharm Rev 2020; 25(1):23–25
- Uhlrich S et al. Alternatives to Animal Testing, Springer, 2019, S. 76–82
- EDQM: Replacement, reduction and refinement of animal testing (3Rs): latest achievements
- Business wire: Prellis Biologics, Inc. generates 300 human antibodies that bind the SARS-CoV2 virus; pursues development of a treatment and preventative therapy for COVID-19 infection
- Giese C et al. Immunological substance testing on human lymphatic micro-organoids in vitro. J Biotechnol 2010; 148(1):38–45
- Goyal G et al. Lymph node follicle formation and vaccination responses reconstituted in vitro in a human Organ Chip. bioRxiv 2019; doi: 10.1101/806505:806505
- Byers AM et al. In vitro antibody response to tetanus in the MIMICTMsystem is a representative measure of vaccine immunogenicity. Biologicals 2009; 37(3):148–151
- Signorazzi A et al. In vitro assessment of tick-borne encephalitis vaccine: Suitable human cell platforms and potential biomarkers. ALTEX 2021; doi: 10.14573/altex.2010081
- Russo G et al. In Silico Trial to test COVID-19 candidate vaccines: a case study with UISS platform. bioRxiv 2020; doi: 10.1101/2020.05.06.080630:2020.05.06.080630
- Viviani L et al. Global harmonization of vaccine testing requirements: Making elimination of the ATT and TABST a concrete global achievement. Biologicals 2020; 63:101–105
- AFSA: Vaccines regulatory alignment
- Halder M et al. Recommendations of the VAC2VAC workshop on the design of multi-centre validation studies. Biologicals 2018; 52:78–82
- Norwig J. Mikrobiologische und biologische Methoden des Europäischen Arzneibuchs. Bundesgesundheitsbl 2014; 57(10):1158–1168
