Prostate cancer: animal experiments and animal-free research
Prostate cancer is one of the most common types of cancer in men. In 2022, 74,895 men were diagnosed with the disease in Germany (1). When detected early, prostate cancer is usually very treatable, whereas advanced cases can have severe consequences. Medical research is therefore focused on developing better diagnostic methods and more effective treatments. This often involves animal experiments. This article explains why such experiments are not effective and how modern, human-focused methods can contribute to better medical advancements.
The prostate, a glandular structure located below the bladder, plays a crucial role in the production of seminal fluid. When prostate cells begin to multiply uncontrollably, this can lead to the formation of a tumor. Prostate cancer is one of the most common cancers in men, particularly in advanced age. The disease usually develops slowly and often goes unnoticed in its early stages. Possible symptoms include frequent or difficult urination, a weak urine stream, blood in the urine or semen, and pelvic pain. However, in many cases, no symptoms appear for a long time, which is why prostate cancer is often only detected during preventive check-ups.
In early stages, the prognosis of prostate cancer is generally very good, and the chances of recovery are high. However, advanced tumors may spread (form metastases) and become more difficult to treat. Therefore, early detection is crucial for improving the outcome. Regular screening is recommended from the age of 45 (1).
Treatment options
Not every case of prostate cancer requires immediate treatment. Since prostate cancer often grows slowly, an approach known as "active surveillance" may be sufficient. This involves regularly monitoring the progression of the disease rather than starting treatment right away.
For faster-growing or more advanced tumors, treatment depends on several factors, including the stage of the tumor, the patient’s age, and overall health. Common treatment options include:
- Surgery: In cases of localized prostate cancer, the complete removal of the prostate (prostatectomy) can be a curative procedure.
- Radiation therapy: Used when surgery is not an option or after surgery to destroy any remaining cancer cells.
- Hormone therapy: Since prostate cancer growth is often stimulated by male sex hormones (androgens), reducing these hormones can slow tumor growth. Hormone therapy is particularly effective for advanced cancer that has not yet metastasized to other organs.
- Chemotherapy: Primarily used for advanced or metastatic prostate cancer, especially when other treatments are ineffective. However, its effectiveness varies significantly, and in late stages, the impact is often limited, with many patients experiencing a decline in quality of life due to the treatment.
Despite the range of treatment options available, there is still a clear need for new therapies that are more effective and produce fewer side-effects. The development of these treatments continues to involve animal experiments.
Animal experiments
Animal experimentation is still considered the so-called gold standard in cancer research - including prostate cancer research. In these experiments, prostate cancer is predominantly artificially induced in rodents such as mice and rats. The preference for rodents in cancer research is mainly due to their small size and low maintenance costs. Another advantage of mice specifically is that genetic manipulation techniques are highly developed for them, as they are among the most commonly used animals in animal experiments.
Various methods are used to artificially induce prostate cancer in animals. Many of these techniques are also used in research on other types of cancer and are described in more detail in our article ´Cancer: animal experiments and animal-free research´(2).
Chemical induction
One method used to induce prostate cancer in animals is the administration of chemicals that trigger tumor formation. Certain substances are used to alter DNA, promoting the growth of cancer cells. As early as 1937, an experiment was published in which the carcinogenic chemical benzpyrene was injected into the prostates of rats to induce cancer (3).
Hormones can also cause prostate cancer. In some cases, a combination of hormone treatment and chemicals is used to specifically stimulate cancer growth. A commonly used substance is N-Methyl-N-Nitrosourea (MNU), which can cause cancer in various organs, including the prostate. In one experiment, rats were first given a daily injection of a drug that blocks male hormones for 21 days. They were then administered testosterone, followed by an injection of MNU into the abdominal cavity. Two weeks later, researchers implanted testosterone-releasing devices to further promote tumor growth (4).
The chemical induction of tumors is a lengthy process, and in many cases, the desired tumor does not form at the expected location.
Genetic modification
One of the common methods used to induce prostate cancer in animals is genetic modification. In this approach, animals - mostly mice - are either modified with a gene that promotes the growth of prostate cancer cells or genes are removed that normally suppress tumor growth. These genetic modifications are designed to specifically affect the prostate, ensuring that the tumor develops in the intended location rather than in other organs.
A well-known model is the TRAMP model (Transgenic Adenocarcinoma of the Mouse Prostate). In this model, a genetically introduced protein (SV40 T-antigen) leads to spontaneous cancer development in the mouse prostate (5). As early as 12 weeks, the mice show early precancerous changes, and after about 30 weeks, they develop aggressive tumors that spread throughout the body. A similar approach is used in the Hi-Myc mouse model, where the MYC gene, a known driver of tumor growth, is overexpressed. These mice develop early tumor stages within a few weeks, and malignant prostate tumors form within three to six months (6).
Another frequently used model is the Pten-knockout model. In this case, the Pten gene, which normally prevents uncontrolled cell growth, is genetically deactivated. Without this protective gene, mice develop prostate cancer within just a few months (7).
Transplantation of cancer cells
In xenotransplantation, human prostate cancer cells are transplanted into mice to study how tumors grow in a living organism and how they respond to treatment . This method requires mice with weakened immune systems, so that their bodies do not reject the foreign human cells.
A common technique is subcutaneous implantation, where human prostate cancer cells are injected under the skin of the mouse. Within a few weeks, a tumor develops, which can be easily palpated and measured from the outside (8). In some cases, stromal cells derived from the prostate are also injected along with the cancer cells (9). However, the environment in which the tumor grows differs significantly from the natural environment of the human prostate. Additionally, prostate cancer cell lines are often used for transplantation. These cell lines were originally derived from a human tumor but have been cultivated in laboratories - or sometimes in animals - for many generations. As a result, the cells undergo changes over time and no longer fully resemble the original tumor cells.
Mice are injected with prostate cancer cells under the skin. Within a few weeks, a tumor develops. ©adobestock/Hyungkeun
Another technique is subrenal transplantation, where prostate cancer cells are mixed with specific reproductive tract cells from rats and transplanted under the protective membrane of a mouse’s kidney. This is intended to create tissue structures that resemble the human prostate. Even human prostate tissue has been transplanted using this method (8).
In the orthotopic xenograft model, cancer cells are injected directly into the prostate of a mouse, allowing a tumor to grow in its natural location. However, tracking tumor growth in this model is more challenging, which is why imaging techniques such as MRI are often used. Additionally, animals are frequently killed to analyze tumor development in their prostates. Besides conventional tumor cell lines, researchers increasingly use patient-derived tumor cells to grow human tumors in animals. Cancer cells isolated from patients’ blood can also be expanded in the lab and then transplanted into mice. However, these patient-derived tumors grow in mouse tissue, altering their characteristics compared to real patient tumors (8,10).
A major issue with models that rely on injecting human tumor cells is that mice must have a defective immune system, so they do not reject the foreign human cancer cells. This means these models fail to account for how the immune system influences tumor growth or responds to therapies. To address this issue, researchers sometimes use mouse-derived cancer cells instead. Additionally, humanized mice are being developed, which contain human immune cells. This is achieved by transplanting human stem cells, which can then develop into different types of immune cells (2).
Metastasis experiments
To simulate the progression of prostate cancer, animals are injected with cancer cells into their bloodstream, allowing them to spread to other organs via the circulatory system. These experiments are conducted to study the metastatic potential of tumor cells and assess the effectiveness of cancer therapies. Depending on the type of injected cells and the injection method, metastases develop in different organs. For example, injecting prostate cancer cells into the heart of mice leads to the formation of bone metastases (11).
Other „animal models“
Chicken embryos are also used in cancer research (12). In these experiments, a hole is cut in the shell of fertilized chicken eggs, and cancer cells or tumor tissue are applied to the membrane of the chicken embryo. The eggs are then incubated further, allowing the tumor to grow and cancer cells to spread within the embryo.
A particularly problematic issue is that experiments on chicken embryos do not require approval in Germany. The EU Directive 2010/63/EU, which aims to protect animals used in experiments, only covers mammalian embryos, not avian embryos (13). This means that experiments on chicken embryos are officially considered a “humane alternative” to animal experiments (14).
In addition to chicken embryos, dogs have also been used in prostate cancer research. For instance, dogs' immune systems were suppressed with medication, and then canine prostate cancer cells were injected into their prostates. Tumor growth was monitored, and the dogs were euthanized once the tumors reached a certain size or their health deteriorated (15).
Despite the widespread use of various “animal models”, there are numerous points of criticism that question their translatability to humans.
The failure of animal-based prostate cancer research
Despite – or rather because of – numerous so-called animal models, the development of new treatment options for prostate cancer remains extremely difficult. Although research on "animal models" has been ongoing for decades, the development of new cancer drugs still ranks among the areas with the lowest success rates. In oncology, the failure rate of animal-based drug development exceeds 95% (16). Particularly revealing is the fact that the highest rate of clinical trial failures occurs after Phase II testing – precisely at the point when the effectiveness of a potential drug is first tested in humans (17). This demonstrates that efficacy found in animal experiments cannot be reliably translated to humans.
Thus, "animal models", such as the xenograft mouse model, are seen by many scientists as a major obstacle in the development of new therapies. The main points of criticism on animal-based methods in the context of prostate cancer research are summarized below.
Biological differences between species
A fundamental problem with animal experiments is that animals often react differently to medications and treatments than humans. For example, animals may metabolize certain tumor drugs differently or can be less sensitive to them. These differences in drug absorption and processing lead to many medications that seem promising in animal experiments but fail to show the desired effect in subsequent clinical trials in humans.
Additionally, the prostate in rodents is structurally very different from that in humans. While the human prostate is a single gland with several zones - such as the peripheral zone, where prostate cancer most commonly develops, and the transition zone, often affected by benign prostatic hyperplasia - the prostate in mice and rats looks entirely different. It consists of four separate lobes: the anterior gland, as well as the upper, lateral, and lower prostates, which are grouped around the neck of the bladder (18).
Differences in tumors
When cell lines are used for tumor-inducing injections, they differ significantly from the cancer cells that grow in the human body. On one hand, these cells change over time due to long-term cultivation in the laboratory; on the other hand, they originally stem from a single cancer cell. As a result, they cannot reflect the genetic diversity and heterogeneity of a real tumor (8). This means that the results of drug testing on these cells hardly translate to humans, or the drugs may only kill a portion of the tumor cells, leaving the remaining cells to regrow the tumor. The transferability is even worse when mouse cancer cells are used instead of human cells (19).
Even when tumor cells that were directly obtained from patients are used to induce prostate cancer in mice, they behave differently than they would in the human body. There is evidence that certain genetic alterations that occur in human tumors are lost in mice. This suggests that the mechanisms controlling tumor growth and development differ between mice and humans (10).
Differences in the tumor microenvironment
Among the most important cell types in the tumor microenvironment are immune cells, cells lining blood vessels (endothelial cells), supporting cells of blood vessels (pericytes), and connective tissue cells. These cells are crucial for the growth and spread of tumors and can promote or influence cancer. As a result, they are increasingly being considered as potential targets for new cancer therapies. However, the environment in which tumors grow in mice differs from that in human tumors. This applies to the “animal models” where prostate cancer is cultivated under the skin or in the kidney, as well as to tumors that are located in the prostate of rats. The surrounding cells are rodent cells and communicate with each other differently than human cells do. As a result, the interaction between cancer cells and their environment cannot be accurately reproduced in animal experiments (8).
When mice with a defective immune system are used, the influence of the immune system on the tumor cannot be observed. However, the immune system plays a crucial role in tumor development and therapy.
Due to the limited suitability of animal experiments, there is a considerable increase in the development of animal-free methods. By using human cells and data, species differences - which lead to poor translatability - can be avoided.
Animal-free research methods
In recent years, animal-free research has made significant advancements. New technologies and innovative approaches not only help to eliminate animal experiments but also improve our understanding of human diseases and generate results that are more transferable to humans.
The following section highlights some of the most promising animal-free methods in prostate cancer research.
In-vitro testing using cell cultures
In in-vitro tests, human cells are studied directly in the laboratory. Typically, human prostate cancer cell lines or cells obtained from patient-derived tumors are cultivated on plastic surfaces, usually forming a single-layer cell culture. These simple cell cultures are quick and easy to use, making them a common tool for testing the effects of potential drugs.
However, these tests do not replicate the three-dimensional growth of tumors, nor do they account for the complex interactions between tumor cells and surrounding cells or structures.
Organoids and 3D-cell cultures
Organoids are miniaturized organs grown from human cells that can mimic the characteristics of real organs. Tumor organoids are increasingly used in cancer research, as they allow for a more precise study of tumor development and provide a more realistic platform for drug development.
In organoids, cells develop within a three-dimensional environment, replicating key tumor characteristics such as oxygen deprivation within the tumor. Organoids can be created from a single cell type or multiple cell types, enabling researchers to study cell interactions within a 3D model. Patient-derived tumor organoids are even more complex and realistic, containing a greater variety of cell types. This method is increasingly used in personalized medicine, as organoids can reflect individual drug responses (20).
By using induced pluripotent stem cells (iPSCs) - which are reprogrammed from normal body cells and can form almost any cell type - prostate organoids can also be developed. These organoids form glandular structures, resembling the natural prostate and containing key cell types, such as hormone-producing cells. They also express typical prostate markers, including the well-known prostate-specific antigen (PSA) (21). These organoids offer a promising tool for advancing prostate cancer research. Additionally, they can be combined with modern gene-editing technologies, such as CRISPR/Cas9, to introduce targeted genetic modifications. With these advanced models, drugs can be tested more effectively, paving the way for tailored cancer therapies (22).
Organ-on-a-chip systems
Organ-on-a-Chip models consist of small microchips that combine microfluidic technology with 3D cell cultures to realistically replicate the tumor environment. Tiny channels within the chip simulate key processes such as blood flow, nutrient supply, and drug transport.
Microfluidic-based systems are particularly useful in cancer research, as they can better mimic the complex tumor microenvironment. They also allow for the consideration of essential physiological factors such as mechanical stress, fluid flow, and pressure. Additionally, these microfluidic systems support personalized medicine for prostate cancer by enabling precise drug delivery to patient-derived tumor cells (e.g., tumor organoids).
These models offer significant advantages in prostate cancer research. One study, for example, used a microfluidic system to grow tumor organoids composed of prostate cancer cells (PC-3), blood vessel cells, and bone cells—allowing researchers to replicate the environment of bone metastases. Experiments using a special organ-chip format have shown that 3D tumor models in these systems exhibit less necrosis (cell death), reduced expression of certain stress-related genes, and better cell growth. Moreover, their structure and properties remain stable, enabling them to demonstrate more realistic responses to chemotherapy (23).
3D-printed models
3D-printed models are also being used for prostate cancer research. One example is a 3D-printed model that combines human osteoprogenitor cells (precursors of bone cells) with prostate cancer cells. For this model, a scaffold structure coated with calcium phosphate is first created via 3D printing to simulate bone tissue. The scaffold is then seeded with human osteoprogenitor cells, allowing them to differentiate and form human bone tissue. This model enables researchers to study interactions between prostate cancer cells and bone tissue over a period of up to 12 weeks, providing insights into bone metastasis. Additionally, it serves as a platform for testing drug efficacy (24).
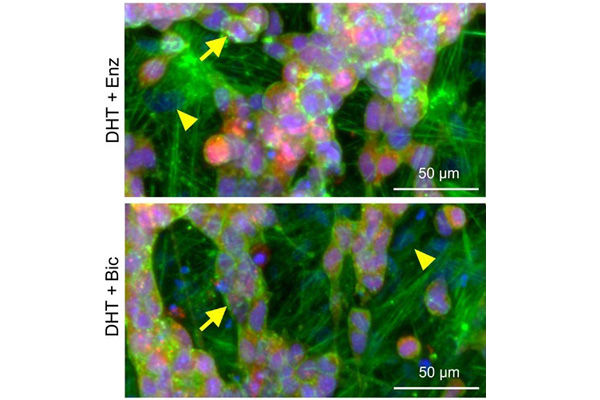
3D-printed human model shows the formation of bone metastases. Source: Bock et al. (2021) (24).
Computer-assisted models and AI
By using computer models, researchers can simulate tumor growth and the effects of medications. Artificial intelligence (AI) is already being used to analyze MRI images and to classify tumors histopathologically.
In the future, AI could also be used to create individual patient simulations, known as "digital twins." These virtual twins could help to develop personalized medical treatments and test the effects of drugs without putting humans or animals at risk (25).
Additionally, AI could help to better understand the mechanisms behind drug resistance or effectiveness. It could also predict which patients would benefit most from a particular drug. AI can thus support nearly all aspects of drug development - from identifying new therapeutic targets and virtual drug screening to predicting pharmacokinetics (how the body absorbs and metabolizes a drug) and potential side effects (8).
Other animal-free methods
Research can also be conducted directly on human tumor tissue without the need to create organoids. For this, tumors that have been removed from patients can be sliced into thin sections and kept alive in a nutrient medium. These slices contain not only tumor cells, but also other cell types arranged exactly as they were in the original tumor.
Etiology and prevention
The causes of prostate cancer are not yet fully understood, but several risk factors play a role. These include genetic predisposition, environmental factors, and lifestyle. Men with a family history of prostate cancer have a higher risk of developing the disease. Regular screening is recommended for men aged 45 and older.
An unhealthy diet, obesity, and lack of physical activity are also considered risk factors. Maintaining a healthy weight and engaging in regular exercise may reduce the risk of prostate cancer, whereas sexually transmitted infections appear to increase it (1). Some studies suggest that a diet rich in vegetables and an adequate intake of antioxidants and vitamins may offer protection against various types of cancer (26).
Modern animal-free research methods could help improve our understanding of prostate cancer development, identify risk factors, and enable effective prevention strategies. More information on prevention can be found in our article ´Cancer Prevention´ (26).
Conclusion
Animal experimentation has long been considered the gold standard in prostate cancer research. However, differences in species and the disparities between artificially induced tumors in animals and those naturally occurring in humans prevent the results from being reliably translatable. As a result, animal experiments are scientifically unsuitable for prostate cancer research and hinder the development of new therapies.
Animal-free methods, such as human organoids, in vitro tests, computer models, and AI, offer better translatability, making research more efficient while preventing animal suffering. The development of these technologies must continue to be advanced, and existing methods must be consistently utilized. This is the only way to find better and more targeted treatment options for prostate cancer.
04.02.2025
Dr. rer. nat. Johanna Walter
References
- Prostate cancer (prostate carcinoma) (in German), Zentrum für Krebsregisterdaten, 14.10.2024
- Walter, J. Cancer: Animal experiments and animal-free research. Doctors Against Animal Experiments, 09.03.2023
- Moore Robert A. et al. Production of tumors of the prostate of the white rat with 1:2-Benzpyrene. The American Journal of Cancer 1937; 30(4):731–741
- Faustino-Rocha A.I. et al. Evolution of models of prostate cancer: Their contribution to current therapies. Anticancer Research 2023; 43(1):323–333
- Hurwitz A.A. et al. The TRAMP mouse as a model for prostate cancer. Current Protocols in Immunology 2001; 45(1)
- Ellwood-Yen K. et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003; 4(3):223–238
- Liu S. et al. A novel controlled PTEN-knockout mouse model for prostate cancer study. Frontiers in Molecular Biosciences 2021; 8:696537
- Sailer V. et al. Experimental in vitro, ex vivo and in vivo models in prostate cancer research. Nature Reviews Urology 2023; 20(3):158–178
- Chung L.W.K. et al. Co‐inoculation of tumorigenic rat prostate mesenchymal cells with non‐tumorigenic epithelial cells results in the development of carcinosarcoma in syngeneic and athymic animals. International Journal of Cancer 1989; 43(6):1179–1187
- Ben-David U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nature Genetics 2017; 49(11):1567–1575
- Thomsen M.K. et al. Pre-clinical models to study human prostate cancer. Cancers 2023; 15(17):4212
- Chu P.-Y. et al. Applications of the chick chorioallantoic membrane as an alternative model for cancer studies. Cells Tissues Organs 2022; 211(2):222–237
- Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes
- Ranjan R.A. et al. The chorioallantoic membrane xenograft assay as a reliable model for investigating the biology of breast cancer. Cancers 2023; 15(6):1704
- Keller J.M. et al. A novel canine model for prostate cancer. The Prostate 2013; 73(9):952–959
- Biomedtracker: Why are clinical development success rates falling? 2024
- Sharpless N.E. et al. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Reviews Drug Discovery 2006; 5(9):741–754
- Lamb D.J. et al. Challenges in prostate cancer research: Animal models for nutritional studies of chemoprevention and disease progression. The Journal of Nutrition 2005; 135(12):3009S-3015S
- Voskoglou-Nomikos T. et al. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 2003; 9(11):4227–4239
- Walter J. Personalized medicine in cancer therapy, Doctors Against Animal Experiments, 08.03.2024
- Hepburn A.C. et al. Propagation of human prostate tissue from induced pluripotent stem cells. Stem Cells Translational Medicine 2020; 9(7):734–745
- Buskin A. et al. Engineering prostate cancer in vitro: what does it take? Oncogene 2023; 42(32):2417–2427
- Payne M.C. et al. Microwell‐based flow culture increases viability and restores drug response in prostate cancer spheroids. Biotechnology Journal 2023; 18(6):2200434
- Bock N. et al. In vitro engineering of a bone metastases model allows for study of the effects of antiandrogen therapies in advanced prostate cancer. Science Advances 2021; 7(27):eabg2564
- Non-fiction: Digital human twins on the rise, News, Doctors Against Animal Experiments, 11.07.2024
- Cancer prevention, Doctors Against Animal Experiments, 2024
