Breast cancer: animal experiments and animal-free research
Breast cancer is the most common cancer in women worldwide. According to the German Centre for Cancer Registry Data, breast cancer was diagnosed in 74,500 women and 690 men in Germany in 2022. Over the course of their lifetime, one in eight women will be confronted with a breast cancer diagnosis (1). Although treatment options have significantly improved in recent decades, breast cancer remains one of the leading causes of death among women (2). The complex biology of the disease and its various subtypes make the development of new treatment options challenging. Moreover, research on new therapies is still predominantly based on so-called animal models. This article explores why animal-based breast cancer research is not effective and which modern, human-focused methods are better suited.
Breast cancer arises from uncontrolled cell growth in the mammary glands and can be classified into benign (non-cancerous) and malignant (cancerous) tumors. Malignant tumors have the potential to spread through the lymphatic or blood system and form metastases in other parts of the body. Understanding the mechanisms that lead to the development and spread of breast cancer is crucial for developing effective therapies.
Available treatment options
The treatment of breast cancer typically includes surgery, chemotherapy, radiation therapy, hormone therapy, targeted therapies, or combinations of these approaches. The choice of therapy depends on the subtype of breast cancer, its stage, and the individual characteristics of the patient.
- Surgery: The tumor is surgically removed. Depending on its extent, this may involve a breast-conserving surgery or a mastectomy.
- Chemotherapy: This treatment uses drugs to kill cancer cells throughout the body. Unfortunately, it is often associated with severe side effects, as healthy cells can also be damaged.
- Radiation therapy: High-energy radiation is used to target the tumor and inhibit the growth of cancer cells. For example, radiation can shrink a tumor to facilitate its surgical removal.
- Hormone therapy: For tumors that rely on hormones to grow, treatments are available that block hormone activity or reduce hormone production.
- Targeted therapies: These relatively new treatment approaches attack specific molecules responsible for cancer cell growth and spread. Because of their targeted nature, they can reduce systemic side effects on healthy cells (3).
Despite these manifold treatment options, there remains a significant need for the development of new therapies. In advanced cancer cases, where the tumor has already metastasized, survival rates drop significantly. Additional challenges include drug resistance and the often severe side effects of existing treatments.
To develop new and more effective therapies, many researchers are still using animal experiments.
Animal experiments
As in cancer research in general (4), it is predominantly mice who suffer and die in breast cancer research. This is not due to a particular similarity to humans; they are used because they are small, and easy and cost-effective to maintain. Additionally, the techniques for genetic manipulation of mice – for example, to make them more susceptible to cancer or to insert human genes – are far advanced compared to other animal species (5).
Since mice and other rodents rarely develop cancer naturally, researchers must artificially induce breast cancer in these animals. Several methods have been developed for this purpose, which are briefly described below.
Induction of tumors
To induce breast tumors, various chemicals or carcinogenic viruses are administered (5). One example is the chemical 7,12-Dimethylbenz(a)anthracene (DMBA), which is specifically used to trigger breast cancer in mice or rats. DMBA is a potent carcinogen that causes DNA damage. The substance is typically administered to the animals once a week for 4 to 6 weeks, either using a gavage tube or by intraperitoneal injection. Within 150 to 200 days, 30 to 70% of the mice develop breast tumors, some of which metastasize to the lungs (6).
Breast cancer can also be induced by physical methods such as ionizing radiation. For example, whole-body irradiation with X-rays or neutron radiation causes breast cancer in rats (5). Irradiation of mice with a dose of 2 Gray results in breast cancer in 22% of the animals; often, the mice also develop radiation-induced ovarian cancer (7).
Transplantation of cancer cells
The methods of induction described above often lead to the formation of the desired tumors in only a portion of the animals. Additionally, it takes a long time for tumors to develop, and the timeframe can vary significantly. To create faster and more easily integratable "animal models" for experimental planning, methods for transplanting cancer cells have been developed.
Since breast cancer originates from the mammary glands, the cells are preferably injected directly into these glands, where they form tumors. However, this method is technically challenging and allows for the transfer of only a small number of cells. For this reason, cancer cells are often injected into the fat tissue of the breast. Another approach is the injection of cells under the skin, as the resulting tumors can easily be observed and measured from the outside. To specifically induce metastases, breast cancer cells are injected into certain areas of the body. For example, an injection into the tail vein of the animals leads to metastases in the lungs. When the cells are injected directly into the heart, metastases form in the bones and brain (5).
The models can be classified into allografts and xenografts, depending on the origin of the cells. In allografts, mice are injected with cancer cells that also originate from mice. These cancer cells either come from spontaneously developed tumors or from tumors that were artificially induced (as described above). In these cases, a tumor composed of mouse cells grows within a mouse body. However, both the tumor itself and the organism in which it develops differ significantly from the situation in humans.
In an effort to better "model" the human condition in animals, human breast cancer cells are also transplanted into mice. This procedure creates what is known as a xenograft model. Mice with a severely compromised immune system are used for this purpose, as their own immune system would otherwise reject the human cancer cells. Examples include nude mice (lacking T cells), NOD-SCID mice (lacking T and B cells), or NSG mice (lacking T cells, B cells, natural killer cells, and macrophages)(8).
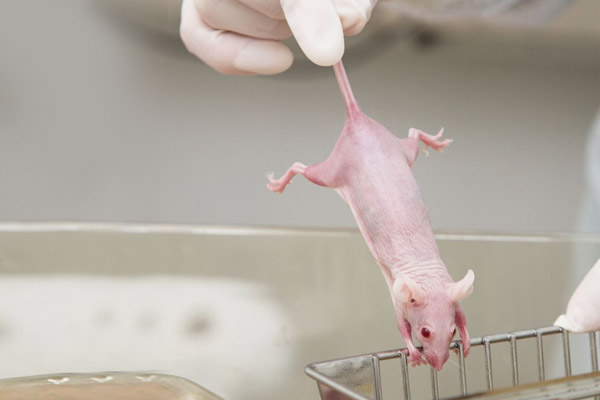
In cancer research, nude mice with a defective immune system are frequently used. Source: adobestock/Vasiliy-Koval.
In many cases, tumor cell lines are used—these are cells originally derived from human tumors that have often been cultivated in the laboratory for decades. However, due to prolonged cultivation under artificial conditions, these cell lines have undergone changes and differ from the tumors that grow in patients in important characteristics. To address this issue, so-called PDX models (patient-derived xenografts) are increasingly being used. In these models, the transplanted cells come directly from tumor samples taken from patients. However, PDX models also have a significant drawback: the microenvironment in which the tumor grows does not match the conditions in the human body. Additionally, the severely compromised immune system of the animals prevents the study of tumor-immune system interactions or the effects of immunotherapies.
This contributes to the limited transferability of animal experiments to humans. Therefore, efforts are being made to "humanize" these animal models, making them more similar to humans. This primarily involves modifying the immune system of the animals by replacing parts of it with human immune components. To achieve this, human immune cells—either in the form of peripheral blood mononuclear cells (PBMCs) or hematopoietic stem cells (HSCs)—are introduced into mice before or after tumor cell transplantation. However, even such humanized models can only partially replicate the human immune system. Moreover, using certain human immune cells can lead to graft-versus-host disease (GvHD), where the implanted human immune cells attack the mouse's own cells. This reduces the animals' lifespan and consequently shortens the duration of studies (9).
Genetically modified "mouse models"
Using genetic engineering methods, specific genes in mice can be deliberately altered to induce tumor formation. This can be achieved either by deactivating genes that normally suppress tumor growth or by inserting so-called oncogenes—genes that promote tumor development—into the mice's genome.
One example of this is the oncogene polyomavirus middle T (PyMT). When this gene is integrated, it leads to particularly aggressive tumor growth. The affected mice develop visible breast tumors as early as 4 to 8 weeks of age, and in 84% to 90% of cases, metastases form in the lungs by 14 weeks of age (5).
Human genes or genetic mutations associated with breast cancer are also frequently introduced into the mouse genome to study the disease.
Other "animal models"
In addition to mice and rats, other animal species such as nematodes, fruit flies, zebrafish, and chickens are also used in breast cancer research (5). The main reason for this is the potential for faster execution of experiments and lower costs. For example, researchers have injected cells from human bone metastases into zebrafish embryos to study the metastatic potential of these cells (10). However, these species differ even more from humans than mice and rats, and many of their genes have no equivalent in humans. This makes the transferability of results to humans even less likely.
Larger animals, such as dogs and non-human primates, in which breast cancer can occur spontaneously like in humans, as well as pigs, have also been proposed and used as models for breast cancer (11-14). Researchers argue that their greater genetic similarity to humans and their larger body size could improve the translatability of the results obtained by using such models.
Even cats have been used as models for breast cancer research. Both spontaneously developed breast tumors and tumors induced by injecting tumor cells into cat embryos have been studied. For example, cells derived from a feline breast tumor were injected into feline embryos while still in the womb. At birth, these cats already had tumors, which metastasized within 6 to 10 weeks. Researchers believe this model could help replicate and study metastatic breast cancer in humans (15).
The failure of animal-based breast cancer research
Animal experiments are supposedly conducted to understand disease mechanisms, develop therapies, and test the safety and efficacy of new treatments before they are evaluated in clinical trials on humans. However, it is precisely in these human trials that the lack of predictive power of animal experiments is exposed.
Despite numerous "animal models" and decades of research, the development of new cancer drugs remains one of the least successful areas in medicine. In oncology, the failure rate of animal-based drug development exceeds 95% (16). Notably, the highest dropout rate in clinical trials occurs after Phase II testing—the stage at which a drug’s efficacy in humans is first assessed (17). This clearly demonstrates that efficacy observed in animal experiments does not translate to humans.
As a result, many scientists view "animal models," such as the xenograft mouse model, as a major obstacle to developing new therapies (17). The main points of criticism on animal-based methods in the context of prostate cancer research are summarized below.
Differences between species
The biology of rodents and their tumors differs significantly from that of humans and human tumors (18). For instance, the full maturation of mammary glands in rodents depends on pregnancy, whereas this is not the case in humans. This affects the presence of multipotent stem cells, which play a role in the development of breast cancer (18).
Biological signaling pathways, gene expressions, or metabolic processes in mice may also function differently than in humans. These differences can lead to variations in the efficacy and toxicity of drugs. For example, mice do not produce a molecule corresponding to human interleukin-8 (IL-8), and the function of the cytokine is likely taken over by other molecules. However, IL-8 plays a significant role in cancer development and has a significant impact on the tumor microenvironment, which cannot be replicated in mice. Other potential target structures for combating cancer may also differ in mice compared to humans, as well as the metabolism of the compound (15). As a result, drugs that are effective and well-tolerated in mice often prove to be ineffective or even harmful in clinical trials.
Additionally, animal experiments are predominantly conducted on genetically uniform mice from inbred strains. This means that the genetic diversity of human patients and individual differences in drug responses cannot be accounted for. Furthermore, the animals are young, which does not reflect the patient population.
Differences within the tumors
Sporadic cancer cases are rare in rodents. The shorter lifespan of rodents means that tumors induced in them must progress quickly in order to be observable in an animal experiment (18).
To achieve this, cancer cells derived from mice are often used, which differ significantly from human tumor cells. For example, about half of human breast cancers are hormone-dependent, whereas the vast majority of murine tumors are hormone-independent and have far fewer hormone receptors than human tumors (19). Although similar morphological patterns can be observed in the tumors of mice and humans, the detailed structure of most mouse tumors does not correspond to the most common types of human breast cancer. Tumors in rats also differ in their detailed histology from both murine and human tumors. Furthermore, the metastasis patterns differ between the species (18). Breast cancer in humans typically spreads through the lymphatic system, starting with nearby lymph nodes. This is followed by distant metastasis, primarily in the bones, brain, liver, and lungs. In contrast, breast tumors in mice metastasize almost exclusively through the bloodstream to the lungs (18).
Even when human cancer cell lines are used, only a few human tumor cell lines are typically employed, which do not represent the vast genetic diversity found in actual tumors in patients. This limitation further diminishes the predictive value of these models for clinical outcomes (17). Human breast tumors vary greatly from one patient to another, and even within a single tumor, there can be substantial differences. This variability cannot be accounted for when using cell line-derived tumors.
Differences in the tumor’s microenvironment
In addition to the tumor itself, its surrounding environment in mice—or other animals—also differs significantly from that in the human body. When human tumor tissue or cell lines are transplanted into immunodeficient mice (such as nude or SCID mice), so-called chimeric tumors are formed, containing a mixture of human tumor cells and murine stroma. Overall, xenografts have fewer stromal cells, and the present stroma is composed of murine tissue. These chimeric tumors can differ greatly in their biology from that of human tumors, leading to unpredictable growth or altered metastatic properties. Another significant limitation common to all xenograft models is the lack of an immune response against the tumor cells. Additionally, the host mice (such as SCID and nude mice) have profound defects in their immune response, which makes the effective testing of immunomodulatory drugs impossible (17).
Therefore, "animal models" do not replicate the complex interactions between the tumor and its microenvironment, which can significantly influence the response to a drug (17).
Although many genetically modified mice have been created, the limitations of all these "mouse models" — including differences in molecular components between species, varying gene expression profiles, and genetic backgrounds — make it impossible to directly apply the findings from these models to drug development. Moreover, most genetically modified mice exhibit a whole-body phenotype, where all tissues and cells carry the same genetic defect. Thus, they do not mimic sporadic tumors caused by a mutation affecting only a single cell in an otherwise normal environment (18).
As early as 20 years ago, Ismail Kola, Senior Vice President for Basic Research at Merck Research Labs, noted that the high failure rates in the development of new cancer drugs are due to the low predictive power of the "animal models" used. The xenograft models frequently used in the pharmaceutical industry, where a tumor cell line that may be of little relevance to tumors growing in the human body is injected into a nude mouse, also contribute to this problem. These models neither reflect the human immune system nor does the artificial placement of the tumor mimic the actual process of tumor development in the body (20).
Trapped in the system of animal experimentation?
What do researchers conclude from all the evidence regarding the failure of animal-based cancer research? Most do not conclude that a shift away from animal experiments and a move toward exclusively human-based methods is necessary. Instead, it is often suggested that the limitations of the various "animal models" can be overcome by combining several models (5). Existing "animal models" should also be further refined. However, the hope that combining different "animal models" can leverage the "advantages" of individual models and bypass their disadvantages must be weighed against the fact that the transferability of animal experiments to humans can only be assessed retrospectively, i.e. only after human trials have already been conducted. This means that combining different models will lead to even more animal experiments without solving the fundamental problem, which is the lack of translatability to humans.
Some researchers argue that xenograft models, despite their poor track record, are still useful for the development of cancer drugs. They claim that drugs that fail in these studies would likely also show no effect in humans. However, this assumption has never been proven and raises the troubling possibility that promising therapeutic agents might be mistakenly discarded in the preclinical phase due to a lack of activity in animal trials (17).
Countless animals live, suffer, and die for questionable research, whose successes fall far behind expectations. On the other hand, patients and their families are waiting for life-saving therapies. However, new medications often offer little more than modest life extension, often at the cost of severe side effects. At the same time, animal experiments are time-consuming and expensive, driving up development costs and needing time.
Therefore, more scientists are realizing that the use of animal experiments in the development of cancer drugs has proven to be problematic and has led to many significant failures (17). Considering this devastating track record, the question arises whether sticking to the animal experiment system is a major contributing factor, or even the root cause, of why many patients are still waiting in vain for new therapies. As a result, an increasing number of scientists are developing animal-free methods for cancer research that are more efficient, ethical, and translatable to humans.
Animal-free research methods
The development of animal-free methods has made considerable progress in recent years and a variety of animal-free approaches for breast cancer research are available. The reference laboratory EURL ECVAM (European Centre for the Validation of Alternative Methods) published a collection of 935 animal-free methods for studying breast cancer in 2020 (21). Our NAT database currently (as of 31.03.2025) contains 219 animal-free methods for breast cancer research (22). The main types of animal-free methods are briefly explained below.
Cell culture experiments
In preclinical cancer research, that is, in the development and testing of potential therapies, cell-based methods are used alongside animal experiments. Simple cell models are often employed, such as human breast cancer cells, which either grow on a cell culture surface or are studied as small mini-tumors in the form of organoids. These models are simple and cost-effective and allow testing whether a potential drug causes cancer cell death (4). However, these systems lack the necessary complexity, as they are solely focused on the tumor cells and do not account for the natural tumor environment, such as neighboring cells.
To overcome these limitations, human-based techniques have been developed that allow consideration of the tumor microenvironment and the systemic interactions of the drug with various organs.
Organoids
Organoids are three-dimensional cell cultures that can be derived from human tissue and replicate the structure and function of organs. Breast cancer organoids enable the study of tumor growth, metastasis, and the effects of drugs in a human context. Particularly promising are patient-derived organoids (Patient-derived Organoids, PDOs), as they originate from primary human tumors, preserving the complex structure and heterogeneity of the tumor. In this process, tumor tissue, typically obtained from surgical excisions or needle biopsies, is broken down into individual cells, which are then cultured to form three-dimensional (3D) structures (23).
Not only tumor organoids can be produced, but also organoids replicating healthy breast tissue (24). For example, human induced pluripotent stem cells (hiPSCs) can be used to generate organoids from breast tissue. These organoids mimic the characteristics of human breast tissue and have even been stimulated to produce milk proteins. The breast organoids derived from hiPSCs can, for example, be used to investigate how various factors influence the development of breast cancer (25).

Breast organoids (bottom) replicate healthy breast tissue (top). Source: Goldhammer et al. Breast Cancer Res. 2019 (24).
It is also possible to model the tumor microenvironment in organoids by integrating additional cell types such as normal breast epithelial cells, fat cells, fibroblasts, or immune cells. This increases the complexity and relevance of the organoid model (23). Overall, breast cancer organoids represent a powerful model system that allows the study of disease mechanisms and the testing of therapeutic responses using exclusively human material.
Organ-on-a-chip systems
This technology uses small devices that simulate human tissue and organ systems. Cells or tissues from different organs – for example, in the form of organoids – are cultured in small chambers, which are connected to each other by fine channels. These channels mimic blood vessels and enable studies to evaluate the metastasis of tumor cells. Organ chips can be used to test the effects of drugs under nearly realistic conditions.
For example, adipose tissue plays a crucial role in breast cancer. Fat cells influence tumor cells by releasing signaling molecules that affect nearby tumor cells and their metabolism. Through these mechanisms, fat cells can contribute to the development, growth, and metastasis of breast cancer (26). Against this background, adipose tissue from human donors can be integrated into organ chips. By using adipose tissue from different donors, varying in age, origin, and nutritional status, it is possible to study how diverse types of fat affect and interact with breast cancer. In 2023, Doctors Against Animal Experiments awarded the Herbert Stiller Prize, worth € 20,000 to Prof. Dr. Peter Loskill from the Eberhard Karls University of Tübingen. In the award-winning project, a breast-cancer-on-a-chip model is being developed. Patient-specific breast cancer organoids are cultured together with adipose tissue in an environment resembling the human breast. Samples from different patients (e.g., healthy, obese, postmenopausal) are used to study specific cancer processes (27).
Additional cells can be integrated, too. For example, human immune cells allow consideration of the influence of the immune system on the tumor and its treatment. The metabolism of drugs and their distribution in various organs and tissues can also be studied in multi-organ chips. Furthermore, organ chips have been successfully used in toxicity testing (28).
3D-printed model systems
Recreating the complex microarchitecture of the breast is crucial for understanding breast cancer. Advances in 3D printing have enabled the development of customizable models, including systems that replicate vascular structures, skin, lungs, kidneys, cartilage, and the brain. In recent years, bio-printed models for breast, brain, ovarian, and skin cancers have been developed and optimized (26). In 3D printing, materials are applied layer by layer to create three-dimensional structures. Materials such as alginate or hydrogels are often used, which are then colonized with cells.
An example is the model developed by Dance et al., which replicates human breast tumors using collagen gels and a stroma of fat cells, as well as stem cells found in adipose tissue. Additionally, the model includes cavities similar to the lymphatic system to study the invasion of cancer cells and metastasis (29). Horder et al., on the other hand, created spheroids from stem cells found in adipose tissue within a hyaluronic acid-based bio-ink. The stem cells were differentiated into fat cells and breast cancer cells were added to model adipose breast cancer tissue (30).
In preclinical studies, 3D-printed models enable the integration of multiple cell types and the replication of physiologically relevant tissue structures. The potential for automating this technology further supports the efficient and reproducible production of the models (26). By integrating various human cells and structures, tumor organoids can be studied in a physiologically relevant environment.
Computer-assisted models and AI
Computational models and Artificial Intelligence (AI) play an increasingly significant role in cancer research. They can be used to analyze data from clinical studies and make precise predictions about the effects of drugs.
Genetic algorithms have already been extensively researched for the diagnosis of breast cancer. Computer-assisted methods and algorithms have also been used to understand the molecular mechanisms of breast cancer, predict drug resistance, and discover new biomarkers for effective treatment (31). AI can, for example, also improve the prediction of response to preoperative chemotherapy (32).
AI is also used in the development of drug structures. Computer-assisted methods such as molecular docking and machine learning are employed to identify potential drug candidates. In-silico studies and deep learning techniques are used to predict molecular mechanisms, pharmacokinetic properties, and the safety of potential drugs (33).
AI thus holds immense potential to improve the detection, diagnosis, and treatment of breast cancer. By analyzing massive amounts of data, AI algorithms can identify patterns and anomalies that may not be detectable by human observers.
More precise weapons in the fight against cancer: targeted therapies
Targeted therapies represent a promising treatment option for breast cancer. These therapies specifically attack tumor cells or inhibit their growth while largely sparing healthy cells. As a result, targeted therapies offer the potential for high efficacy while reducing side effects. However, developing such therapies for breast cancer is challenging, as they aim to block specific molecular signaling pathways or genetic abnormalities that drive tumor growth.
Traditional animal-based research reaches its limitations in this context: Many molecular mechanisms involved in tumor development in the human body are either absent or differently regulated in animals, making animal experiments ineffective. Furthermore, many targeted therapies are designed to interact with specific components of the human immune system. Since the immune system of animals functions differently or does not work properly in models such as PDX (patient-derived xenografts), these interactions cannot be adequately tested in animal experiments.
For these reasons, human-based research methods are indispensable for the development of targeted therapies. Patient-specific models, such as breast cancer organoids derived from tumor samples, offer a promising solution.
Patient-derived breast cancer organoids are also useful for determining which therapy a specific tumor responds to best. This allows for the selection of the most effective treatment for each individual patient. Here, the advantage of animal-free methods becomes particularly evident: They not only provide results that are translatable to humans but also enable personalized medicine tailored to individual patients. For more information on personalized cancer therapy, see our article ´Personalized medicine in cancer therapy´ (34).
Prevention
A central goal of breast cancer research is to understand the causes of the disease. This includes identifying genetic risk factors through molecular analyses. Epidemiological studies have also identified environmental and lifestyle factors associated with breast cancer. These factors can be influenced, forming the basis for breast cancer prevention.
Smoking, alcohol consumption, poor diet, and lack of physical activity are key factors that increase the risk of developing breast cancer. However, these risks can be mitigated through appropriate lifestyle adjustments. Alcohol consumption is a significant and avoidable risk factor for breast cancer, responsible for 7% of new cases (35). Contrary to widespread belief, there is no safe level of alcohol consumption, and abstaining from alcohol has been proven to reduce the risk of breast cancer and other diseases (36). For detailed information on prevention, see our article ´Cancer prevention´ (37).
Conclusion
Breast cancer research is at a turning point. While animal experimentation has long been considered indispensable and is still regarded as the "gold standard," it is becoming increasingly clear that it hinders progress. The high number of ineffective or unsafe drugs entering clinical trials demonstrates the need for better methods to predict efficacy. With technologies such as organoids, organ-on-a-chip systems, and artificial intelligence, scientists can obtain human-relevant insights that will lead to improved treatment options for breast cancer.
31.03.2025
Dr. rer. nat. Johanna Walter
References
- Centre for Cancer Registry Data, Breast cancer, 30.09.2024
- Federal Statistical Office, Leading causes of death, female, 2023
- ONKO Internetportal, Targeted therapy for breast cancer (in German), 27.07.2022
- Walter J. Krebs: Cancer: Animal experiments and animal-free research, Doctors Against Animal Experiments, 09.03.2023
- Zeng L. et al. Breast cancer animal models and applications. Zoological Research 2020; 41(5):477–494
- Plante I. Methods in Cell Biology, Elsevier, 2021, Vol. 163, 21–44
- Rivina L. et al. Mouse models for radiation-induced cancers. Mutagenesis 2016; 31(5):491–509
- Chakrabarti R. et al. Mouse models of cancer, Methods in Molecular Biology, Springer New York, 2015, Vol. 1267, 367–380
- Li Z. et al. Application of animal models in cancer research: recent progress and future prospects. Cancer Management and Research 2021; Volume 13:2455–2475
- Mercatali L. et al. Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. International Journal of Molecular Sciences 2016; 17(8):1375
- Nguyen F. et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: natural history and prognostic factors. Breast Cancer Research and Treatment 2018; 167(3):635–648
- Razavirad A. et al. Canine mammary tumors as a potential model for human breast cancer in comparative oncology. Veterinary Medicine International 2024; 2024(1):9319651
- Deycmar S. et al. Spontaneous, naturally occurring cancers in non-human primates as a translational model for cancer immunotherapy. Journal for Immunotherapy of Cancer 2023; 11(1):e005514
- Mondal P. et al. Large animal models of breast cancer. Frontiers in Oncology 2022; 12:788038
- Smith B.F. et al. An in utero allotransplantation model of metastatic breast cancer in the cat. In Vivo (Athens, Greece) 2003; 17(1):35–39
- Biomedtracker, Why are clinical development success rates falling?, 29.04.2024
- Sharpless N.E. et al. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Reviews Drug Discovery 2006; 5(9):741–754
- Kim J.B. et al. Models of breast cancer: is merging human and animal models the future? Breast Cancer Research 2003; 6(1):22
- Nandi S. et al. Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proceedings of the National Academy of Sciences 1995; 92(9):3650–3657
- Kola I. et al. Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery 2004; 3(8):711–716
- European Commission, Joint Research Centre, Advanced non-animal models in biomedical research, Publications Office, 2020
- NAT Database
- Li X. et al. Advances in breast cancer organoid for individualized treatment. Organs-on-a-Chip 2023; 5:100028
- Goldhammer N. et al. Characterization of organoid cultured human breast cancer. Breast Cancer Research 2019; 21(1):141
- Qu Y. et al. Differentiation of human induced pluripotent stem cells to mammary-like organoids. Stem Cell Reports 2017; 8(2):205–215
- Hamel K.M. et al. Adipose tissue in breast cancer microphysiological models to capture human diversity in preclinical models. International Journal of Molecular Sciences 2024; 25(5):2728
- Herbert Stiller Prize award ceremony for research without animal experiments in Tübingen, News, Doctors Against Animal Experiments, 17.10.2023
- Ewart L. et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Communications Medicine 2022; 2(1):154
- Dance Y.W. et al. Adipose stroma accelerates the invasion and escape of human breast cancer cells from an engineered microtumor. Cellular and Molecular Bioengineering 2022; 15(1):15–29
- Horder H. et al. Bioprinting and differentiation of adipose-derived stromal cell spheroids for a 3D breast cancer-adipose tissue model. Cells 2021; 10(4):803
- Khan A. et al. Editorial: breast cancer resistance, biomarkers and therapeutics development in the era of artificial intelligence. Frontiers in Molecular Biosciences 2022; 9:1034990
- Taylor C.R. et al. Artificial intelligence applications in breast imaging: current status and future directions. Diagnostics (Basel, Switzerland) 2023; 13(12):2041
- Nafissi N. The application of artificial intelligence in breast cancer. Eurasian Journal of Medicine and Oncology 2024; doi: 10.14744/ejmo.2024.45903:235–244
- Walter J. Personalized Medicine in Cancer Therapy, Doctors Against Animal Experiments, 08.03.2024
- World Health Organization, Alcohol is one of the biggest risk factors for breast cancer, 20.10.2021
- World Health Organization, No level of alcohol consumption is safe for our health, 04.01.2023
- Walter J. Prevention of Cancer, Doctors Against Animal Experiments, 08.04.2024
