Antibodies: The future is cruelty-free
Although efficient methods for the animal-free production of antibodies are available, it is estimated that more than 1 million animals die for antibody production every year in the EU alone. (1) This is in direct violation of EU Directive 2010/63/EU, which prohibits the use of animals if animal-free methods are available. (2) In response to this situation, the EU reference laboratory for alternatives to animal testing (EURL ECVAM) issued a recommendation against the further use of animals for the production of antibodies. (1) This article provides insights into the different methods for the production of antibodies and demonstrates why the animal-free production of antibodies is not only beneficial for animal welfare, but also for the progress of science and medicine - and thus for all of us.
Whether for COVID-19 or doping tests, for cancer or multiple sclerosis medication - antibodies are indispensable in diagnostics, therapy, and research. Antibodies are the central defense molecules of our immune system. Through precise binding towards their respective antigen, they neutralize pathogens and foreign substances, or label them so they can be recognized and eliminated by immune cells. Thus, antibodies can be used to treat various diseases such as infections and cancer. Their precise binding to their target molecule - the so-called antigen - is also used in diagnostics and biomedical research. Due to their broad applicability and further fueled by medical progress, there is an enormous need for more and more antibodies: new diseases are being discovered, new virus variants have to be detected, and novel or improved therapies are supposed to benefit people.
To meet this need, modern in-vitro methods such as the phage display technology, which was awarded the Nobel Prize for Chemistry in 2018, have been developed. Nevertheless, most antibodies are still developed or produced in-vivo, i.e. in the living organism, which comes at the cost of animal suffering and a number of disadvantages for scientists and patients.
Conventional antibody development: not human(e)
The starting point for the development of conventional, animal-derived antibodies is the exploitation of an animal's immune system through immunization. For this purpose, the animal is repeatedly injected with the antigen, i.e. the substance against which an antibody is to be developed. The antibodies are produced by specific blood cells named B cells. Many different B cells produce one specific antibody each, resulting in an immune response consisting of a mixture of different antibodies against different portions of the antigen, the so-called polyclonal antibodies.
Antibody-rich sera obtained from animals and polyclonal antibodies purified from these sera are still used today. In Germany, 58,699 animals were used in 2020 for the production of blood-based products, which include both sera and polyclonal antibodies. Of these, 56,230 were rabbits. (3) According to our investigations, the main user of rabbits for the production of blood-based products in Germany is the company Siemens Healthineers in Marburg. (4)

58,699 animals were used in Germany for the production of blood-based products, which include sera and polyclonal antibodies, in 2020. 96% of these were rabbits. In addition, 2,469 other animals, among them mainly mice, sheep, rats, and dogs, had to suffer for the manufacture of these products. (3)

From 2015 to 2018, between 260,000 and 295,000 animals per year were used for the production of blood-based products in the EU (the figures for 2019 and 2020 are not yet available). German animal testing laboratories made a significant contribution to these figures (23 to 34%).
Examples of products manufactured by immunization are the immunosuppressive thymoglobulin, which is produced in rabbits, or antivenoms directed against snake venoms, which are obtained from the blood of immunized horses. A drug for diphtheria is also made from horse blood. (4) In addition to ethical concerns about the use of horses, which are often kept in deplorable conditions, these sera also pose a risk to humans. The human immune system might react to the animal-derived antibodies and after the administration of the diphtheria antitoxin, a life-threatening serum disease occurs in about 5% of cases. (5)
Another problem associated with polyclonal antibodies is that they do not only bind to the intended antigen but might also bind to other molecules with similar structural elements. This is problematic not only for therapeutic use, but also for research, as it reduces the validity of results obtained when using polyclonal antibodies. In addition, polyclonal antibodies are only available - in a more or less consistent form - from one single animal, and even then, there are batch-dependent variations in quality. After the death of the respective animal, a polyclonal antibody is no longer available in the same composition. If scientists are aware of this problem at all, it requires that every single batch of a polyclonal antibody has to be tested and validated carefully. Otherwise, it is questionable whether published results can be reproduced. (6)
In contrast to polyclonal antibodies, monoclonal antibodies bind to a single, precisely defined structural element of the antigen. Monoclonal antibodies are produced by single B cells. To obtain these cells, animals - usually mice - are repeatedly injected with the antigen. Then the animals are killed and B lymphocytes are isolated from their spleens. Each of these cells produces a single monoclonal antibody. From the large number of B lymphocytes obtained from each spleen, the cells that produce an antibody with the desired properties are identified in a laborious screening process.
To make the monoclonal antibodies permanently available, the spleen cells can be fused with cancer cells. The resulting hybridoma cells contain the spleen- cell-derived genetic information for the expression of the monoclonal antibody and combine it with the cancer cell’s unlimited ability to divide itself. (7)
Once a hybridoma cell is established, it can be kept in culture for any length of time and releases the antibodies into the cell culture supernatant. At least in the initial phase of the cultivation, fetal calf serum, which is obtained from the still beating heart of the calf when a pregnant cow is slaughtered, is often added to the cell culture media. (8) However, the fetal calf serum can be replaced by human platelet lysate, which can be obtained from unused components of blood donations. (9)
Depending on the amount of antibody required, the hybridoma cells can be cultivated in different scales by using small reaction vessels, larger bottles or bioreactors, which are available in a wide range of sizes up to several 1,000 liters. The antibody can be produced in the bioreactor under precisely defined and reproducible conditions. Antibodies produced under such conditions are therefore of consistent and high quality.
Mice are abused as living bioreactors
Although monoclonal antibodies can be produced in-vitro, i.e. in cell culture in easily scalable bioreactors, antibodies are still produced using the extremely cruel ascites method (see also Table 1). To do this, mice are first injected with a mineral oil into their abdominal cavity, which irritates the peritoneum.
Hybridoma cells are then introduced into the abdominal cavity of the mice. The hybridoma cells multiply uncontrollably and form a tumor which produces large amounts of the monoclonal antibody and releases it into the ascites fluid. This fluid is obtained with a cannula by so-called puncturing, which can be done about twice a week for 2 to 3 weeks. Some experimenters use the trivializing and inappropriate term "milking" (10) for this highly stressful animal experiment. After that, the tumor has grown so big that the animals have to be killed.
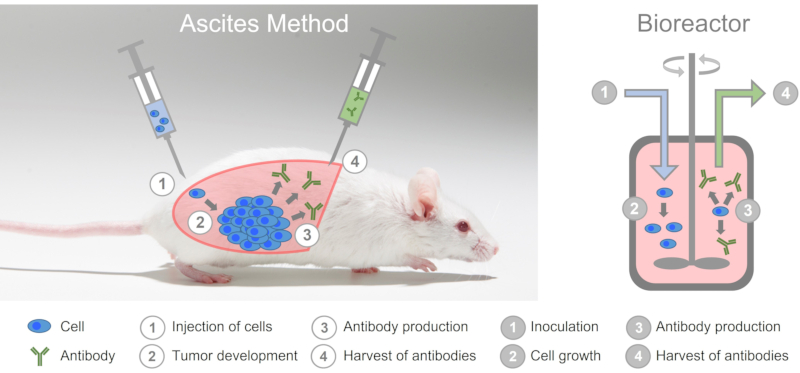
In the ascites method, mice are abused as living bioreactors.
The animals suffer great pain during the experiment. (11) Already in 1998, the ECVAM declared that in-vitro methods are available for the production of antibodies that are equal or even superior to the ascites method and that the use of the ascites method should therefore be limited to a few exceptions that, however, need to be well-founded. (12) Nevertheless, 1,456 mice were used in the ascites method in Germany in 2019 (13) and in 2020, 1,050 mice were used to produce monoclonal antibodies. (3)
Humanization of antibodies: the long way from animal to patient
Irrespective of their production (in-vivo or in-vitro), animal-derived monoclonal antibodies are recognized as foreign by the human immune system. To prevent this, monoclonal antibodies for therapeutic applications need to be humanized in a complex process to make them more similar to human antibodies. For this purpose, the constant portions of the animal antibodies, which are not involved in binding to the antigen, are genetically replaced by the corresponding human sequences.
The genetic information for the production of the humanized monoclonal antibodies is then transferred into so-called production cell lines, which consequently produce the antibody and release it into the cell culture supernatant. Most production cell lines are also of animal origin. The CHO (Chinese Hamster Ovary) cell line, which is most commonly used for antibody production, was obtained from the ovary of a Chinese dwarf hamster. Even if the genetic information for the production of humanized antibodies is introduced into these cells and the antibodies produced in this way consist of the human basic structure, they still differ from those produced by the human immune system. In addition to the amino acid sequence, an antibody also contains sugar residues. These are attached to the antibody by the cell after the readout of the genetic information and the production of the corresponding amino acid sequence.
Thus, humanized antibodies produced in animal cells are equipped with sugar residues specific for the respective animal species, which differ from those of humans in their structure, the so-called glycosylation pattern. (14) These differences can be sufficient for the human immune system to recognize the antibodies as foreign and combat them.
For example, administration of cetuximab, an antibody that is used for the treatment of colorectal cancer and is produced in animal-derived production cells, induced immune responses in patients. These adverse effects range up to anaphylaxis, a potentially life-threatening allergic reaction as a consequence of the use of the animal production cell line. (16) To prevent this, a number of human production cell lines have been developed. In these, the antibody can be produced with the appropriate human glycosylation pattern. (14)
Antibodies can also be produced without animals
It seems not only cruel, but also very laborious to have antibodies produced by the immune system of animals, kill the animal, isolate and screen the B cells, determine the genetic information for antibody production, and then genetically engineer them to humanize them, in order to then insert them into production cells, which - if they are not of human origin - again lead to deviations from the antibodies produced by the human immune system. And in fact, there is an easier method available. Instead of using a laborious detour via the animal, antibodies can be developed completely animal-free and humanely.
The most widely used method for developing non-animal antibodies is phage display technology. (17) For this, B cells are isolated from a donor's blood, and the genetic information for the production of the antigen-binding regions of the antibodies is obtained. This genetic information is incorporated into the genome of phages, which are viruses that infect bacteria. The phages then produce antibody fragments and attach them to their surface.
Comparison of human immune system and phage display

Left: Human B cells display various antibodies on their surface. When they come into contact with the antigen, plasma cells are formed from the B cells decorated with the appropriate antibody. The plasma cells produce the antibody.
Right: In phage display technology, DNA is extracted from B cells and transferred into phages. The resulting phages carry antibody fragments on their surface. Addition of the antigen allows the selection of phage with corresponding antibody fragments. The DNA for producing the antibody fragment is then isolated from the phage and transferred to production cells.
In this way, so-called phage libraries are accessible, in which each phage presents a different antibody fragment on its surface and at the same time contains the genetic information for the production of this specific antibody fragment. The antibody fragments that bind to a desired antigen can now be systematically selected from these libraries. In this selection process, the antibodies can already be adapted to their later application (see also Table 2). For example, specificity can be guided by performing counter-selections in which antibody fragments that bind to structures similar to the antigen are removed. In addition, it is possible to use already "pre-immunized" antibody libraries. For example, recovered COVID-19 patients have been used as donors to develop a phage library to obtain antibodies for COVID-19 therapy. (18)
In particular for therapeutic applications, a number of antibodies developed via phage display have already been approved and many more are in clinical trials. (19) An example is Humira, a drug used to treat rheumatoid arthritis with which the pharmaceutical company AbbVie generated sales of more than 20 billion US dollars in 2021. (20) While the phage display method is already established and accepted in therapeutic applications, there is still considerable resistance to phasing out animal-based antibody development, especially in biomedical research.
Aptamers as a cruelty-free substitute for antibodies
All methods presented in this article so far depend on an immune system, either directly or indirectly, and result in antibodies as products of cells. But there are alternatives to antibodies. Those can be produced completely animal- and cell-free. One example are the so-called aptamers.
In contrast to antibodies, aptamers do not consist of amino acids, but of nucleotides, the basic building blocks of DNA. Unlike genomic DNA, aptamers do not fold into the well-known double helix, but into complex three-dimensional structures. These structures allow the precise binding to a desired molecule, which is comparable to the binding between an antibody and its antigen.
Aptamers are developed from artificially prepared DNA libraries via an in-vitro process named SELEX (Systematic Evolution of Ligands by Exponential Enrichment). (21) The libraries consist of oligonucleotides containing a randomized region, in which the 4 basic building blocks of DNA, the so-called nucleobases, are lined up in a random order. This gives the libraries an extraordinary variety of individual sequences: With a number of 40 randomly arranged nucleobases, theoretically 440 individual, different sequences can be obtained. That is a quadrillion (1024), or 1,000,000,000,000,000,000,000,000 different DNA molecules. In comparison, it is estimated that the human immune system has a repertoire of “only” one trillion (1018) different antibodies, (22) which represents one millionth of the diversity of DNA libraries.
Each individual DNA sequence in the library folds into its own 3D structure. In order to obtain aptamers that bind to a desired target molecule, the library is brought into contact with this target molecule. The sequences that bind to the target molecule are isolated and amplified using the polymerase chain reaction (PCR). The result is a mixture of different oligonucleotides that already exhibit a certain affinity for the target molecule. This mixture is again brought into contact with the target molecule, with the individual sequences competing for binding to the target structure. The sequences with the better binding properties prevail in this competition. This process is repeated several times until sequences with the desired affinity are obtained. After the last round of selection, the obtained aptamers are sequenced and can then be produced by chemical synthesis. As with the phage display method, counter-selections can also be carried out within the SELEX procedure in order to influence the specificity of the aptamers.
The resulting aptamers can have affinities similar or even superior to those of antibodies. A major advantage is that, in contrast to antibodies, aptamers can be produced chemically using solid-phase synthesis. Since aptamers are produced entirely without animals, cells, and animal products, contamination of the aptamers with viruses or potentially immunogenic substances originating from animals or cell culture is completely avoided. This is an advantage in particular for therapeutic applications. An aptamer was approved in 2004 for the treatment of wet age-related macular degeneration, a disease of the eye. Other aptamers are in clinical testing. (23)
Why is there so much resistance to non-animal antibodies and their alternatives?
Animal-derived antibodies are associated with specificity problems, which jeopardize the gain in knowledge, and with batch-to-batch variations, which impede their reproducible use. Many scientists seem to have come to terms with these shortcomings, or perceive them as general and unavoidable problems of antibodies. They are unaware of new techniques, such as phage display technology, or underestimate the power of these methods, and continue to cling to traditional processes. Therefore, it is also due to the researchers' hostility to innovation that even such cruel methods as the ascites method are still used.
For many experimenters, keeping animals is easier than learning and using modern in-vitro methods. As a cruel consequence, mice are misused as living and sentient bioreactors for antibody production. Not because there are no animal-free methods, but because it is more convenient to keep the already established animal-based method than to switch to modern and animal-free methods. In addition, from the perspective of many researchers, there is no need for action. Animal-based research is still funded - mostly using taxpayers' money - and can be published in renowned journals. And this despite the fact that the EU Directive 2010/63/EU on the protection of animals used for scientific purposes actually prohibits animal testing if equivalent non-animal methods are available. (2)
How can we succeed in phasing out animal-based antibodies?
In its recommendation, the EU reference laboratory for alternatives to animal testing (EURL ECVAM) explicitly points out that phage display technology is a valid method for the animal-free development of antibodies and that projects using animals for antibody development should therefore be rejected by the approval authorities. The production of antibodies by the ascites method should no longer be allowed under any circumstances. ECVAM has defined a series of measures to promote the timely phase-out of animal-derived antibodies and to break the resistances outlined in the last section. (1)
Funding institutions that support research projects with public or private funds should stop to support projects that involve the development of animal-derived antibodies. In addition, the funding institutions should promote the development of animal-free antibodies by distributing the funds accordingly. The scientists themselves are also addressed by ECVAM. They should preferably use animal-free antibodies to pursue their research goals, and academic institutions should establish appropriate animal-free methods.
Antibody manufacturers and their distributors are encouraged to replace animal-derived antibodies in their catalogs with non-animal antibodies, and should establish and pursue concrete plans to phase out animal-derived antibody production in a timely manner.
Finally, ECVAM also addressed publishers, editors, and reviewers, who should ensure that the sources of antibodies are clearly described in publications. Work using animal-derived antibodies despite the availability of animal-free alternatives should no longer be published.
The ECVAM is therefore addressing all relevant stakeholders and recommends a catalog of measures. Work containing animal-derived antibodies should no longer be approved, funded, and published in the future.
We will continue our work to ensure that regulatory authorities, funders, and publishers heed these recommendations.
Conclusion
The detour via an animal immune system when producing antibodies for human applications is laborious, unnecessary, and risky, since the antibodies of humans and animals are different. A human immune system can be imitated in-vitro with modern display methods, and the use of human production cell lines in modern bioreactors can result in human and humane antibodies, which can be used therapeutically for the benefit of patients and cause no animal suffering. Thus, the use of animals to produce antibodies is not only ethically unacceptable, but also contradicts the EU directive, according to which the use of animals is only justified if there are no animal-free alternatives. In addition, antibodies produced without animals have superior properties, which are urgently needed in biomedical research.
The insistence on animal-derived antibodies is outdated, hinders scientific and medical progress, and thus harms not only the animals who lose their lives, but all of us.
References
(1) Barroso, J. et al. EURL ECVAM Recommendation on non-animal-derived antibodies; Publications office of the European Union, 2020
(2) Richtlinie 2010/63/EU des europäischen Parlaments und des Rates vom 22. September 2010 zum Schutz der für wissenschaftliche Zwecke verwendeten Tiere
(3) Bericht des BfR vom 16.12.2021: Zahlen zu den im Jahr 2020 verwendeten Versuchstieren
(4) Wenzel, E. V.et al. Human antibodies neutralizing diphtheria toxin in vitro and in vivo. Sci. Rep. 2020, 10 (1), 571
(5) Kupferschmidt, K. Scientists find way to make diphtheria treatment without injecting horses with toxin. Science 2020. https://doi.org/10.1126/science.aba9484.
(6) Goodman, Simon. L. The antibody horror show: an introductory guide for the perplexed. New Biotechnol. 2018, 45, 9–13
(7) Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256 (5517), 495–497
(8) www.fks-frei.de
(9) Kirsch, H. M. Nachhaltige in vitro Kultivierungsansätze in der Zellkulturtechnik: Xenofreie Kultivierungsoptimierung mit Thrombozytenlysat. Dissertation, Leibniz Universität Hannover, 2021
(10) Richtlinie ‚Herstellung von Monoklonalen Antikörpern 5.01‘, Bundesamt für Lebensmittelsicherheit und Veterinärwesen BLV, Schweiz, Juli 2017
(11) Fischer, R. W. & Ferber, P. C. Monoclonal antibodies comparative methods for in vitro production. ALTEX - Altern. Anim. Exp. 1992, 9 (1), 15–24
(12) ECVAM statement on the scientific acceptability and practical availability of in vitro methods for the production of monoclonal antibodies. 14. Mai 1998
(13) BMEL 8.12.2020: Versuchstierdaten 2019.
(14)Fliedl, L. et al. Human cell lines for the production of recombinant proteins: on the horizon. New Biotechnol. 2015, 32 (6), 673–679
(15) Fischer, J. et al. Alpha-Gal-Syndrom: Ein Überblick zum klinischen Bild und zu pathophysiologischen Konzepten. Hautarzt 2022, 73 (3), 195–200
(16) Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N. Engl. J. Med. 2008, 358 (11), 1109–1117
(17) https://www.aerzte-gegen-tierversuche.de/de/sonstige/antikoerper-aus-phagen
(18) Dübel, S. et al. COR-101, ein menschlicher Antikörper gegen COVID-19. BIOspektrum 2021, 27 (1), 46–48
(19) Frenzel, A. et al. Designing human antibodies by phage display. Transfus. Med. Hemotherapy 2017, 44 (5), 312–318
(20) https://de.statista.com/statistik/daten/studie/547194/umfrage/umsatz-des-pharmaunternehmens-abbvie-mit-dem-arzneimittel-humira/
(21) Zhuo, Z. et al. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18 (10), 2142
(22) Briney, B. et al. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature 2019, 566 (7744), 393–397
(23) Li, Z. et al. Advances in screening and development of therapeutic aptamers against cancer cells. Front. Cell Dev. Biol. 2021, 9, 662791
